| Title | EFFECTS OF BIO-NORMALIZER (A FOOD SUPPLEMENTATION) ON FREE RADICAL PRODUCTION BY HUMAN BLOOD NEUTROPHILS, ERYTHROCYTE AND RAT PERITONEAL MACROPHAGES |
|---|---|
| Year | 1995 |
| Author | James Akira Osato, PhD, Ludmila G. Korkina, PhD, DSC, Librado A. Santiago, PhD, and Igor B Afanas’ev, PhD, DSC |
| Publisher | Nutrition |
Effects of Bio-Normalizer (A Food Supplementation) on Free Radical Production by Human Blood Neutrophils, Erythrocyte and Rat Peritoneal Macrophages
James Akira Osato, PhD, Ludmila G. Korkina, PhD, DSC, Librado A. Santiago, PhD, and Igor B Afanas’ev, PhD, DSC
Osato Research Institute, Gifu, Japan and the Russian Institute of Pediatric Hematology, Moscow, Russia
Date accepted: 15 July 1995
ABSTRACT
Bio-normalizer, a natural Japanese health food prepared by the fermentation of Carica papaya, exhibits therapeutic properties against various pathologies including tumors and immunodeficiency. To understand the mechanism of bio-normalizer’s therapeutic effects, we studied its action on the production of active oxygen species in cell-free systems (the Fenton reaction, the xanthine-xanthine oxidase system, and the hydrogen peroxide-hypochloride or hydrogen peroxide–horseradish peroxide systems) and by human blood neutrophils and erythrocytes and rat peritoneal macrophages. Bio-normalizer efficiently inhibited the formation of oxygen radicals in cell-free systems and partly decreased spontaneous and menadione-stimulated superoxide production by erythrocytes, but manifested both stimulatory and inhibitory effects on oxygen radical release by dormant and activated phagocytes (neutrophils and macrophages). We suggest that Bio-normalizer is able to enhance the intracellular production of innocuous superoxide ion and, at the same time, to diminish the formation of reactive hydroxyl radicals, perhaps by the inactivation of ferrous ions, the catalysts of the superoxide-driven Fenton reaction. We also propose that the normalization of an organism’s superoxide level is one of the molecular mechanisms of Bio-normalizer activity. Nutrition 1995; 11:568-572
Key words: bio-normalizer, free-radicals, neutrophils, macrophages
INTRODUCTION
Bio-normalizer, a traditional natural Japanese health food prepared by the fermentation of Carica papaya, exhibits therapeutic effects in various human diseases. It was previously proposed that Bio-normalizer efficiency in the treatment and prevention of severe pathologies is linked to its free radical scavenging properties such as the scavenging of hydroxyl radicals1 and the inhibition of lipid peroxidation.2 There is now a large body of evidence that free radical scavengers and antioxidants may inhibit neoplastic events at cellular and molecular levels,3 prevent various environment-associated pathologies,4and diminish other disorders mediated by free radicals. It is also well established that the most important source of reactive oxygen species in mammalians is phagocytosing cells such as circulating neurophils and tissue macrophages
Correspondence to: Igor B. Afanas’ev, PhD, Research Institute, Nauchny pr. 14A, Moscow 117820, Russia
Therefore, we have studied for the first time the effects of Bio-normalizer on oxygen radical production by human blood neutrophils and erythrocytes and by rat peritoneal macrophages. We found that Bio-normalizer both inhibited and stimulated the release of oxygen radicals by phagocytes, which is supposed to be an origin of bio-normalizer’s in vivo biological activity. In addition, we showed that Bio-normalizer scavenged free radicals produced in some cell-free systems.
MATERIALS AND METHODS
Chemicals
Bio-normalizer was produced by Sun-O International Inc., Gifu, Japan, by the fermentation of Carica papaya tropical herbal plants. A major component of Bio-normalizer is a mixture of plant and yeast glycopolysaccharides. In addition, Bio-normalizer contains amino acids (for example, tryptophan, leucine, and glutamic and aspartic acids), vitamins B-6 and C, glucose, and the protease-antiprotease system of papain. Under our experimental conditions, the suspension of Bio-normalizer granules was prepared daily in Hank’s balanced salt solution (HBSS). In most experiments, Bio-normalizer particles were sedimented by centrifugation after a 2-h incubation in HBSS, and clear supernatant containing the soluble Bio-normalizer (SBN) fraction was used in experiments. SBN contained all the above cited compounds excluding insoluble, high molecular weight glycopolysaccharides.
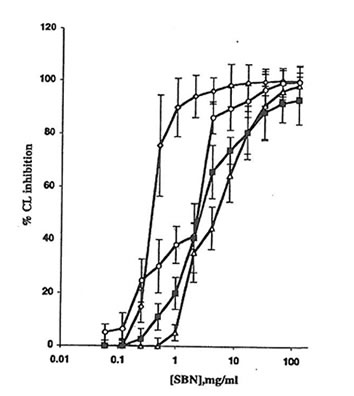
Fig. 1. Effect of soluble Bio-normalizer (SBN) on the production of active oxygen species in cell-free systems: à, the Fenton reaction (H2O2 + FeSO4), every point is an average of four experiments;D, hydrogen peroxide-hypochloride system, every point is an average of three experiments;m, hydrogen peroxide-horseradish peroxidase system, every point is an average of five experiments; and n, xanthine-xanthine oxidase system, every point is an average of four experiments. Experimental details are presented in the MATERIALS AND METHODS section. CL, chemiluminescence.
Cytochrome c, NADPH, lucigenin, luminol, menadione, phorbol myristate acetate (PMA), zymosan A, latex particles (1mm), Ficoll-Hypaque, Monoprep, and bovine superoxide dismutase (SOD, EC 1.15.1.1) were from Sigma Chemical Co.(St. Louis, USA). Dextran sulfate and sodium metrizoate were from Pharmacia (Uppsala, Sweden).
Blood Drawing and Cell Isolation
Venous blood (2ml) was obtained from healthy subjects after their informed consent. Heparin-treated blood was carefully layered on a dextran-metrizoate mixture (2 ml, 2:5 vol:vol) and incubated at room temperature for 30 min. Then, the leukocyte-rich top layer was placed on 1 ml Ficoll-Hypaque (d=1.119) and centrifuged at 400 x g for 30 min. The neutrophil-containing pellet was washed twice in cold HBSS and resuspended in a minimal essential medium (MEM). Cell suspension contained >95% neutrophils as measured by differential cell counts with a Coulter counter. Neutrophil viability was 90-98% as assessed by the exclusion of 0.2% trypan blue dye.
Erythrocytes were collected from the bottom layer after dextran-metrizoate sedimentation, washed 3 times with 0.1 mol potassium-sodium phosphate buffer/L (pH 7.3), diluted with this buffer to the 2% hematocrit, and stored at 4ºC until analyzed. Cells were counted by the Coulter method.
Animal and Macrophage Preparation
Adult male Wistar rats were injected intraperitoneally with 10 ml HBSS and killed 5 min later. The peritoneal lavage fluid was removed, placed on top of Monoprep solution (d = 1.068), and centrifuged at 400 x g for 30 min. The upper portion containing mononuclear cells was washed twice with cold calcium- and magnesium-free HBSS and resuspended in MEM supplemented with 1% heat-inactivated calf serum. A differential cell count was performed microscopically using Giemsa staining:about 80% of cells were marophages and monocytes and the others were lymphocytes, mast cells, and erythrocytes; >95% of cells excluded trypan blue.
Measurement of the Production of Active Oxygen Species
The production of active oxygen species in Fenton reaction and by the xanthine-xanthine oxidase, hydrogen peroxide-horseradish peroxidase (HRP), and hydrogen peroxide-hypochloride systems was measured by luminol- and lucigenin-amplified chemiluminescence in the chemiluminiscence unit of an LKB luminometer (Model 1251, Wallan, Finland) at 25ºC.
For the Fenton reaction, FeSO4 (2 mmol/L) and luminol (0.1 mmol/L) were incubated with or without Bio-normalizer in 0.125 mol phosphate buffer/L (pH 7.4). Next, the reaction was started by adding hydrogen peroxide (1 mmol/L) and the chemiluminescence intensity was registered continuously.
For measurement in the xanthine-xanthine oxidase system, xanthine (0.1 mol/L) and lucigenin (10 mmol/L) were incubated with or without Bio-normalizer in 0.125 mol Na2CO3/L (pH 7.8) and the reaction was started by adding xanthine-xanthine oxidase (0.5 U/mg). In the hydrogen peroxide-HRP and hydrogen peroxide-hypochloride systems, luminol (0.1 mmol/L) and HRP (0.12 mg) or NaCIO (1 nmol/L) were incubated with or without Bio-normalizer in 0.01 mol Tris-HCl buffer/L and the reaction was started by adding hydrogen peroxide.
For the measurement of superoxide production by erythrocyte, an erythrocyte suspension (20ml) and cytochrome c (50 mmol/L) were incubated with or without bio-normalizer in 0.1 mol potassium phosphate buffer/L (2 ml) with or without menadione (10 mmol/L) at room temperature for 60 min. After the sample was centrifuged, the light absorbance of the clear supernatant was measured at 550 nm on a Beckman DU 600 spectrophotometer (St. Louis, MO, USA). Superoxide content was calculated with the extinction coefficient of reduced cytochrome c equal to 21000 mol .L-1 .cm-1.
To measure superoxide production by neutrophils and macrophages and NADPH oxidase activity,5 we incubated a cell suspension (5 x 105 cells) and cytochrome c (50 mmol/L) with or without Bio-normalizer in HBSS and continuously recorded the light absorbance at 550 nm. The reaction was started by adding PMA (10 ng/ml), stopped at the maximal rate by the addition of 0.5% Triton X-100, and restored 1min later by the addition of NADPH (250 mmol/L).
The production of active oxygen species by neutrophils and macrophages was measured by the chemiluminescence method with lucigenin as a specific chemiluminescence probe for the detection of superoxide ion6 and luminol as the chemiluminescence probe for detecting hydroxyl and hydroxyl-like free radicals.7 Cells (1 x 105) with or without Bio-normalizer were mixed with HBSS (0.9 ml) containing 50 mmol/L luminol or lucigenin. The cell-containing mixture was incubated at 37ºC and mixed continuously for 5 min and spontaneous chemiluminescence was registered. Then, 50 ml of activator (PMA, opsonized zymosan, or latex particles) was added and the maximal chemiluminiscence response to the activator was measured.
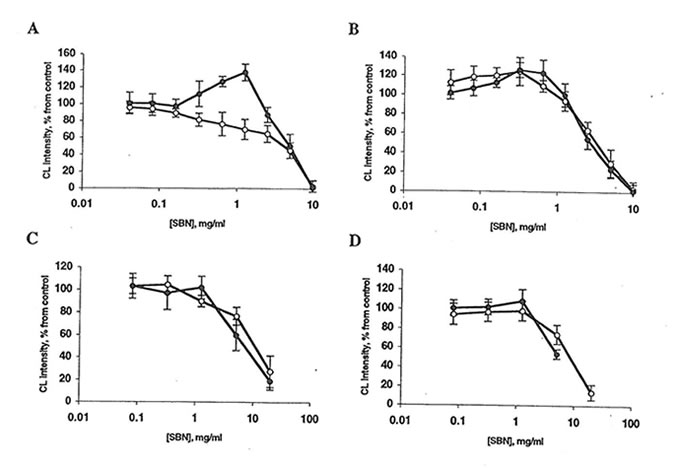
Fig. 2. Effect of Bio-normalizer and soluble Bio-normalizer (SBN) on luminol-amplified chemiluminescence (CL) of rat peritoneal macrophages. Every point is an average of four experiments. l, Bio-normalizer, or n, SBN. A: Spontaneous CL. Basal CL is 20-70 mV/106 cells. The stimulator’s effect of 1.25 mg Bio-normalizer is significantly different from control (p<0.05). B: CL activated by phorbol myristate acetate. The basal CL level is 250-400 mV/106 cells. C: Zymosan-activated CL. Basal CL level is 700-800 mV/106 cells. D: Latex-activated CL. Basal CL is 1100-1400 mV/106 cells. In all cases, the inhibitory effects of 5-20 mg Bio-normalizer/ml or SBN are significantly different from control (p<0.01).
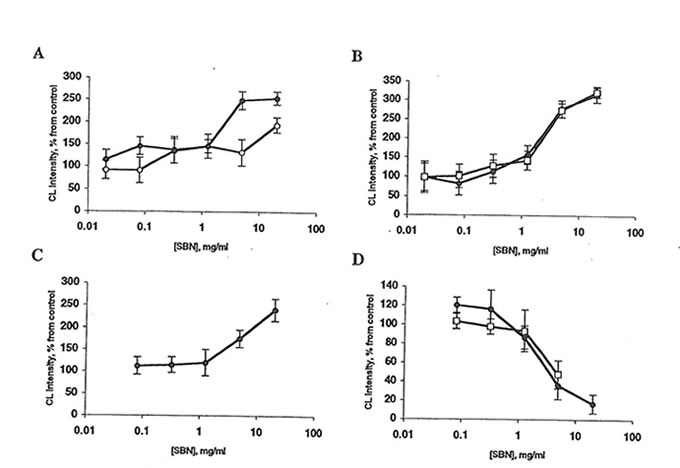
Fig. 3 Effect of Bio-normalizer and soluble Bio-normalizer (SBN) on lucigenin amplified chemiluminescence (CL) of rat peritoneal macrophages. Every point is an average of four experiments. l, Bio-normalizer; or n, SBN. A: Spontaneous CL. Basal CL level is 2-10 mV/106 cells. The stimulatory effects of 5 mg Bio-normalizer/ml and 20 mg SBN/ml are significantly different from control (p<0.01 and 0.05, respectively). B: CL activated by phorbol myristate acetate. Basal CL level is 25-60 mV/106 cells. The stimulatory effects of 5 mg Bio-normalizer/mlor SBN are significantly different from control (p<0.05). C: Zymosan-activated CL. Basal CL level is 30-80 mV/106 cells. The stimulatory effect of 20 mg Bio-normalizer/ml is significantly different from control (p<0.05). D: Latex-activated CL level is 100-150 mV/106 cells.The inhibitory effects of 5 mg Bio-normalizer/ml Bio-normalizer or SBN are significantly different from control (p<0.05).
REFERENCES
- Santiago, L.A., Osato, J.A., Hiramatsu, M. et al. Free radical scavenging action of Bio-catalyzer a.r no.11 (Bio-normalizer and its by-product. Free Radic Biol Med 1991;11:379
- Santiago, L.A, Osato, J.A., Liu, J., et al. Age-related increase in superoxide dismutase and thiobarbituric acid-reactive substances:effect of Bio-normalizer in aged rat brain. Neurochem Res 1993;18:711
- Perchellet J.P., Perchellet E.M. Antioxidants and multistage carcinogenesis in mouse skin. Free Radic Biol Med 1989;7:377
- Ames BN. Dietary carcinogens and anticarcinogens, oxygen radicals and degenerative disease. Science 1983;221:1256
- Bellavite P, Jones OTC, Cross AR, et al. Composition of partially purified NADPH oxidase from pig neutrophils. Biochem J 1984;223:639
- Gyllenhammar H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods 1987;97:209
- Allen RC, Loose LD. Phagocytic activation of a luminol dependent chemiluminescence in rabbit peritoneal macrophages. Biochem Biophys Res Commun 1976; 69;245
- Adams DO, Hamilton TA Phagocytic cells: cytotoxic activities of macrophages. In: Galin JI, Goldstein IM, Snyderman RI, eds. Inflammation: basic principles and clinical correlates. New York: Raven Press, 1988:471
- Ito M, Karmali R, Krim M. Effect of interferon on chemiluminescence and hydroxyl radical production in murine macrophages stimulated by PMA. Immunology 1985;56:533
- Korkina LG, Afanas’ev IB, Samochatova EV, et al. Release of active oxygen radicals by leukocytes of Fanconi anemia patients. J Leukoc Biol 1992;52:357
- Wing EJ, Ampel NM, Waheed A, et al. Macrophage colony-stimulating factor (M-CSF) enhances the capacity of murine macrophages to secrete oxygen reduction products. J Immunol 1985;135:2052
- Oberley CW, Buetner GR. Role of superoxide dismutase in cancer: a review. Cancer Res 1979;39:1140