| Title | ISOLATION AND IDENTIFICATION OF LACTIC ACID BACTERIA INVOLVED IN THE FERMENTATION OF BIO-NORMALIZER |
|---|---|
| Year | 1998 |
| Author | Randolph Scott Jr., Izumi Inoko, James Akira Osato, Ph.D., and Kazuhiro Takamizawa, Ph.D. |
| Publisher | Research Report No. 8 |
ISOLATION AND IDENTIFICATION OF LACTIC ACID
BACTERIA INVOLVED IN THE FERMENTATION OF BIO-NORMALIZER
Randolph Scott Jr.1,2, Izumi Inoko1,2, James Akira Osato, Ph.D.2, and Kazuhiro Takamizawa, Ph.D.3
1Center for Cooperative Research, Gifu University, 1-1 Yanagido, Gifu City 500-1125 Japan
2Osato Research Institute, 21 Imakomachi, Gifu City 500 Japan
3Department of Bioprocessing, Faculty of Agriculture, Gifu University, 1-1 Yanagido, Gifu City
500-1125 Japan
ABSTRACT
A common strain of lactic acid bacteria was isolated and partially identified from all three related sources—papaya fruit (Carica papaya Linn.) skin, fermented papaya juice and Bio-Normalizer powder which is the final product of natural papaya fermentation. Based on the similar biological activities of Bio-Normalizer and lactic acid bacteria strains in general, one of the health beneficial properties of Bio-Normalizer might be a result of fermentation of lactic acid of bacteria. This is the first report of the presence of lactic acid bacteria in both the fermented papaya juice and the powdered form of Bio-Normalizer. The origin of this fermenting lactic acid bacterium species might be the natural bacterial flora of papaya fruit skin based on the morphological and physiological similarity of the lactic acid bacteria strains isolated from these three sources.
1.INTRODUCTION
Bio-Normalizer is a functional health food product that is derived mainly from the natural fermentation of papaya fruit and other Japanese plant ingredients. Bio-Normalizer’s health- promoting properties such as free-radical regulation and immunomodulation have been characterized quite extensively in recent years1,2. However the mode by which the product is fermented and the role of the fermenting organisms in conferring some of the biological properties of Bio-Normalizer are still being studied at present.
Lactic acid bacteria (LAB) have been known to be present in variety of fermented food products, mainly in fermented milk-based products. Lactic acid bacteria can be found in the mouth and intestinal tract of humans as well as in plant material. Although these may include a number of different bacterial genera and species, one major characteristics of these organisms is the production of lactic acid.
A variety of health benefits have been associated with LAB. LAB has been found to alleviate lactose-indigestion, inhibit the growth of pathogens such as Salmonella typimurium, and metabolize toxins such as aflatoxin B3. One current topic research is the immunomodulation provided by LAB. Researches have shown that LAB can stimulate non-specific immunity particularly the production of human Tumor necrosis factor-a, Interleukin-6 and –10, and Interferon 4.
It has been initially observed that lactic acid bacteria might present in the natural fermentation of papaya juice into Bio-Normalizer since Lactic acid was produced in considerable amounts relative to other organic acids during Bio-Normalizer fermentation 5. It is therefore thought that lactic acid bacteria might be directly involved in the fermentation as well as production of health-beneficial substances in Bio-Normalizer.
In this work, lactic acid bacteria were isolated and identified by morphological, physiological and biochemical means to confirm their involvement in Bio-Normalizer fermentation.
2. MATERIALS AND METHODS
2.1 Samples
Bio-Normalizer (BN) powder and fermented papaya juice were obtained from Osato Bio-Industry Corporation, Manila, Philippines. Fresh unripe green papaya fruit (about 15 cm. Long) was taken from Okinawa, Japan and Manila, Philippines.
2.2 Isolation and Purification of Lactic Acid Bacteria
To isolate lactic acid bacteria from BN, one gram of BN was added to 3-ml. Sterile 1% peptone water aseptically and serial dilutions of 1/10, 1/100, 1/1000 were done with 1% peptone water. Aliquots of 1-ml. from each dilution were inoculated into 20-ml. autoclaved molten MRS agar pH 6.2 and incubated at 20 o C until colonies appeared. MRS medium (Oxoid, Australia) composition (in g/L) is as follows (6): peptone, 10; Lab Lemco powder, 8; yeast extract, 4; glucose, 20; Tween 80, 1-ml.; dipotassium hydrogen phosphate, 2; sodium acetate trihydrate, 5; triammonium citrate; 2; magnesium sulfate heptahydrate, 0.2; and magnesium sulfate, 0.05 To isolate LAB from fermented papaya juice, 1-ml. sample was taken from freshly fermented papaya juice and serial dilutions were also made with 1% peptone water. The 1-ml. aliquots were pour-plated with MRS agar and plates were incubated in 20 o C until colonies appeared. For papaya skin, the above procedure was also performed except that instead of liquid samples, one piece of aseptically cut of about 2 cm. square fruit skin was used as an inoculant in 1% peptone water. Colonies that appeared on the plates were then isolated and purified several times by streaking until no observable differences in colony characteristics were observed on each isolate. All the strains were maintained by weekly subculturing on MRS agar at 20 o C.
2. 3 Gram Staining and Catalase Test
Isolates were observed morphologically by Gram staining and presumptive lactic acid bacteria were further identified through catalase testing. Gram staining was performed using a kit while the catalase test was done by pouring hydrogen peroxide solution (10%) onto the surface of MRS agar plates containing the isolated colonies and observing for bubble formation on the colonies which is an indication that the bacteria possess catalase. Only Gram positive and catalase negative strains were further isolated and identified.
2.4 Carbohydrate Metabolism
To study the carbohydrate metabolism of the presumptive lactic acid bacteria, API 50 CH/CHL (Bio-Merieux, France) was used. Briefly, purity of the isolates was tested by streaking and morphological observation. Strains were cultured in 20 o C for 24 hours and swabs of colonies were picked and resuspended in 5-ml. API 50 CHL (MRS medium with Bromocresol purple) suspension medium. Turbidity was adjusted by visual comparison only using Mc Farland tube standards. API 50 CH strips were then filled with the inoculated API 50 CHL medium and overlaid with mineral oil and incubated for 48 hours. Positive test results corresponding to acidification (bromocresol purple turns to yellow, and bromocresol purple turning into black for the esculin test) were observed after 24 and 48 hours of incubation.
2.5 D/L-Lactic acid production
To determine whether D- or L-Lactic acid as well as to quantify the amount of each produced by the putative lactic acid bacteria, an enzymatic analysis kit (Boehringer Mannheim, Germany) was used.
2.6 Growth Temperature
Purified putative lactic acid bacteria were also cultured at 15 o C and 45 o C to determine whether these strains could survive both at high and low temperature.
2.7 CO2 from Glucose
Homofermentative strains were determine by their evolution of CO2 gas from the anaerobic fermentation of glucose in MRS media.
3.RESULTS
Using MRS agar as medium for lactic acid bacteria selective isolation, a total of 36 distinct colonies were isolated from papaya skin while 11 and 3 colonies were isolated from fermented papaya juice and Bio-Normalizer powder respectively. These isolates were purified by streaking on new MRS agar medium and the resulting cultures were tested for purity and morphology by Gram staining. Catalase test was also performed to initially obtain presumptive lactic acid bacteria strains. The presumptive Gram positive and catalase negative cells obtained were classified based on their colony characteristic (color, texture) and as either rods or cocci based on the similarity of their cell morphology (see Table 1).
Table 1. Characteristics of Gram positive, catalase negative presumptive lactic acid bacteria strains isolated from Papaya skin, fermented papaya juice and Bio-Normalizer.
| Source | Colony type | Morphology | Number/Total |
| Papaya skin | Yellow, sticky | Cocci | 21/36 |
| White, opaque | Cocci | 05/36 | |
| White, milky | Rods | 10/36 | |
| Feremnted papaya juice | White, milky | Rods | 11/11 |
| Bio-Normalizer | White, milky | Rods | 3/3 |
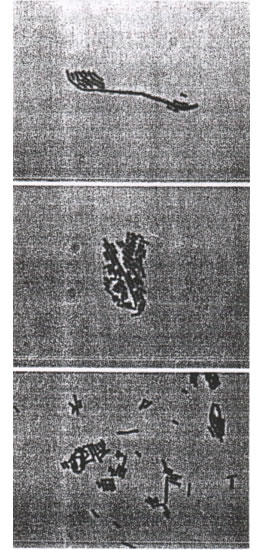
To confirm the identity of all the presumptive lactic acid bacteria based on their physiological utilization of different carbohydrate sources, a total of 10 isolates including representatives for the three sources—namely Bio-Normalizer, papaya juice and papaya skin—were subjected to API 50 CH test. There were four distinct physiological patterns obtained from 10 isolates but only one physiological pattern was common to the three sources. These isolates from the three different sources having this similar pattern of carbohydrate metabolism were all also rods of the same colony and cell morphological characteristics (see FIGURES). API 50 CH was performed at least three times on each isolate to confirm that they all have the same results. These three uniquely similar isolates were further tested to D- and L-lactic acid production as well as growth in 15 o C and 45 o C and production of CO2 upon anaerobic fermentation of glucose. The morphological and physiological characteristics of these isolates are summarized in Table 2. Base on the above characteristics, the three similar isolates were classified as being all Lactobacillus plantarum 7.
Table 2. Summary of the morphological and physiological characteristics of the three common LAB isolates from papaya skin, fermented papaya juice and Bio-Normalizer.
| Gram staining |
+ |
| Presence of Catalase |
– |
| Growth temperature |
15-20 oC, not 45 oC |
| Lactic acid isomer |
D/L |
| CO2 from glucose |
+ (Heterofermentative) |
| Esculin Hydrolysis |
+ |
| Carbohydrates fermented |
Ribose |
FIGURES. Gram positive lactic acid bacteria from papaya isolated from papaya skin, fermented papaya juice and Bio-Normalizer, respectively
4.DISCUSSION
Bio-Normalizer is a functional health food product that is the final powdered form of naturally fermented papaya juice. The fermentation leading to Bio-Normalizer has always been claimed to be mainly a product of yeast fermentation since yeast is added to the fermentation medium, although the presence of other natural flora involved in the fermentation has not yet been clearly excluded. In Bio-Normalizer, yeast counts are generally not much higher than bacterial count8 and that lactic acid was produced in considerably larger amounts than other organic acids such as acetic acid and malic acid 5. The pH of fermented papaya juice as well as dissolved Bio-Normalizer powder also goes down to around pH 4.6, which is close to the usual buffering range of lactic acid. This has led to the inquiry as to how the lactic acid is produced and
what is its relevance in the fermentation of Bio-Normalizer. One group of microorganisms which are common to most naturally fermented food products and which can produce lactic acid during fermentation is generally classified as lactic acid bacteria. In this paper, to determine the source of this lactic acid, the lactic acid bacteria were isolated and purified from the three stages of Bio-Normalizer fermentation, namely: the raw material which is papaya fruit; the fermentation process which is represented by the fermented papaya juice; and the final product which is Bio-Normalizer powder. In all three stages, a common type of lactic acid bacteria, identified by its morphological and physiological properties to be Lactobacillus plantarum was isolated. The isolated L. plantarum in most stages of Bio-Normalizer fermentation might be responsible for the production of considerable amounts of lactic acid.
Bio-Normalizer has been proven to exhibit both free-radical regulation and immunomodulation in clinical trials. Papaya juice on its own has been discovered to have natural free-radical scavenging capacity 9 and thus could partially explain why Bio-Normalizer is free-radically regulating. However, the source of this immunomodulation in Bio-Normalizer has to be further studied. Different types of lactic acid bacteria in food have been known to exhibit several health beneficial activities such as antibacterial, antifungal, antimutagenicity, antitumor, as well as modulation of the immune system. L. plantarum, in particular, has been observed to provide significant adjuvanticity among Lactobacillus sp. 10. The possible involvement of the lactic acid bacteria, L. plantarum in the fermentation process of Bio-Normalizer might open a new avenue for studying the immune stimulation afforded by Bio-Normalizer.
5.REFERENCES
- Osato, J.A., Cuadra, M.S., and Santiago, L.A. (1995). Antioxidant defenses of Bio-Normalizer. Manetic Resonance in Med. 6, 306-308
- Osato, J.A., Afanas’ev, I.B., Korkina, L.G., Santiago, L.A., Mori, A. and Takamizawa, K. (1995). Free radical regulatory and immunomodulatory effects of Bio-Normalizer. In: Ong, A.S.H., Niki E. and Packer, L. [eds.]. Nutrition: Lipids, Health and Diseases. AOCS Press. Illinois, pp. 45-58.
- Kelkar, S.M., Shenoy, M.A. and Kaklij, G.S. (1988). Antitumor activity of lactic bacteria on solid fibrosarcoma-180, and Erlich ascites carcinoma. Cancer letters 42(1-2):73-7.
- Miettinen, M., Vuopio-Varkila, J., Varkila, K. (1996) Production of human Lactic acid bacteria induce tumor necrosis factor alpha, interleukin-6, and interleukin-10. Infection and Immunity 64(12),5403-5405.
- Cuadra, M.S. (1996) unpublished results.
- De man, J.C., Rogosa, M. and Sharp, M.E. (1960) A medium for the cultivation of lactobacilli. Journal of Applied Bacteriology, 23, 130-131.
- Kandler, O. and N. Weiss (1986). Regular, non-sporing, Gram positive rods. In: Bergey’s Manual of Systemic Bacteriology. Vol. 2[Eds.] P.H. Sneath N.S. Nair, M.E. Sharpe, J.G. Holt (1986). Ninth Ed. Williams and Wilkins, Baltimore, MD, USA.
- Osato, J.A. (1996) Biological activity of fermented papaya product. Ph.D. Thesis. United Graduate School of Agricultural Science, Gifu University Science of Biological Resources , Gifu University.
- Webman, E.J., Edlin, G., and H.F. Mower (1988) Free radical scavenging activity of papaya juice. Int. J. Radiat. Biol. 55(3), 347-351.
- Pouwels, P.H., Luer, R..J. and W.J. Boemna (1996). The potential of Lactobacillus as carrier for oral immunization: development of vector systems for targeted delivery of antigens. J. Biotechnol. 26,44(1-3), 183-192.