| Title | HUMAN THIOREDOXIN ADULT T CELL LEUKEMIA DERIVED FACTOR ACTIVATES THE ENHANCER BINDING PROTEIN |
|---|---|
| Year | |
| Author | F Marotta, H Tajiri’, R Barreto, P Brasca, GM Idéo, L Mondazzi, P Safan, J Bobadilla, G ldéo |
| Publisher |
Cyanocobalamin Absorption Abnormality in Alcoholics is Improved by Oral Supplementation with a Fermented Papaya-derived Antioxidant
F Marotta, H Tajiri’, R Barreto2, P Brasca, GM Idéo, L Mondazzi, P Safan3, J Bobadilla2, G ldéo
Hepatogastroenterology Dept. Medical Science Div., S. Giuseppe Hospital, Milano, Italy; 1Research Division Dept., National Shikoku Cancer Center Hospital, Matsuyama, Japan; 2Dept. of Gastroenterology, Instituto Nacional de la Nutrition S. Zubiran, Mexico City, Mexico; 3SFJO Lab., Paris, France
Corresponding Author: Francesco Marotta, M, PhD, Via Pisanello, 4, 20146 Milano, Italy
Fax: +39-024-077243, E-mail: fmarchimede@libero.it
ABSTRACT
Background/Aims: Thirty alcoholic patients and 24 teetotaler dyspeptic patients were considered and underwent baseline blood chemical evaluation and the Schilling test.
Methodology: During gastroscopy,biopsy samples were taken to assay: routine histology, malonyldialdehyde, vitamin E and glutathione concentration and for testing vitamin B12 -Intrinsic Factor binding. Examinations were repeated after 1-week supplementation with Bionormalizer.
Results: Plasma malonyldialdehyde level and lipid hydroperoxides concentration as well as either malonyldialdehyde and xanthine oxidase concentration in the gastric mucosa in alcoholics were significantly higher than in controls and despite unchanged alcohol consumption, significantly decreased after Bionormalizer supplementation. Gastric mucosal glutathione was markedly depressed in alcoholics and partly recovered after Bionormalizer supplementation. Although the alcoholics showed a normal intrinsic factor secretion in the gastric juice, they exhibited a markedly depressed intrinsic factor-cobalamin binding on the “ex vivo” study. Moreover, nearly 23% of them had an abnormal Schilling test. Both these impairments reverted to normal after Bio-normalizer supplementation.
Conclusions: It can be postulated that the antioxidative action played by Bionormalizer, possibly due to its availability substrates for glutathione synthesis as well as to its effects on local oxidative burst from neutrophil, is able to recover a normal cobalamin absorption.
KEY WORDS: Cyanocobalamin absorption; Instrinsic factor binding; Free radical damage; Bionormalizer
ABBREVIATIONS: HepatitisB Virus (HBV ); Antibodies to Hepatits B Virus (anti-HCV); High Performance Liquid Chromatography (HPLC); Superoxide Dismutase (SOD); Dual-lsotope Technique (DIST); Intrinsic Factor (IF);Thiobarbituric Reactive Substances (TBARS)
INTRODUCTION:
Cobalamin malabsorption is likely to be a largely underrated clinical entity and its spectrum covers a wide number of mild-to-undiagnosed cases. It has been shown in humans that the administration of alcohol to healthy volunteers brings about a decrease
in vitamin B12 absorption, as measured by the Schilling test (1). Accordingly, there are albeit scanty reports that alcoholics may have low serum cobalamin levels, which in some cases may revert to normal after prolonged abstinence (2).
Alcoholics’ gastritis has been generally indicated as a background cause of this phenomenon although such clinical entity has been questioned for a long time. Shaw et al (3) have suggested by experimental work that ethanol-induced reactive oxygen species may hamper gastric intrinsic factor binding of B12. We have very recently shown that a novel oral antioxidant, obtained from yeast fermented mixture of medicinal plants (Carica papaya, pennisetum purpureum, Sechium edule) is able to markedly decrease the oxidative stress in alcoholics taking place during the early phase of abstinence (4). The aim of the present study was thus to gain further insight into the presumptive free radical-mediated mechanism of cyanocobalamin malabsorption in a clinical setting of alcoholics while testing at the same time the potential protective effect of Bionormalizer supplementation.
METHODOLOGY
Patients with long-standing elevated alcoholic consumption were considered (> 150g ethanol/day for at least 3 years). All patients underwent a careful personal interview to establish ethanol consumption and drinking behavior. A detailed diet-history method was carried out and nutrient intakes were calculated by using a standardized food-composition table (Table 1). Drinking habit was mostly wine at meal times and between meals. There was no concomitant or prior regular consumption of medications or drugs at the entry into the study. In particular, 9 patients admitted an occasional use of antacids or a short course of H2 blockers or, less frequently, proton-pump inhibitors for the past 18 months.
Nearly 25% of all alcoholics who were initially screened, had been admitted to hospital during the previous 12 months. On these occasions, some of them had received parenteral thiamine administration but none had been given cyanocobalamin supplementation under any formulation. All of them had abnormal liver function tests and with an over 4-fold increase of gamma-glutamyl-transpeptydase value. Two patients were positive for antibodies to hepatitis B virus (anti-HCV) and none had positive markers of ongoing hepatitis B virus (HBV) infection.
There was no evidence of pancreatic insufficiency or intestinal bacterial overgrowth. As a control group, teetotaler subjects undergoing gastrointestinal investigations for non-ulcer-like dyspepsia and devoid of peptic ulcer or any other relevant gastrointestinal disease were considered. Both patients and control subjects had not used vitamin supplements. A positive urease test (FUD test, Medimar, Milano), whose high sensitivity and specificity had already been confirmed in our routine practice was used as an exclusion criteria for all subjects involved in the study. Therefore, 30 patients (25 males and 5 females, mean age: 46 years; range: 42-53years) and 24 control subjects were finally selected for the study.
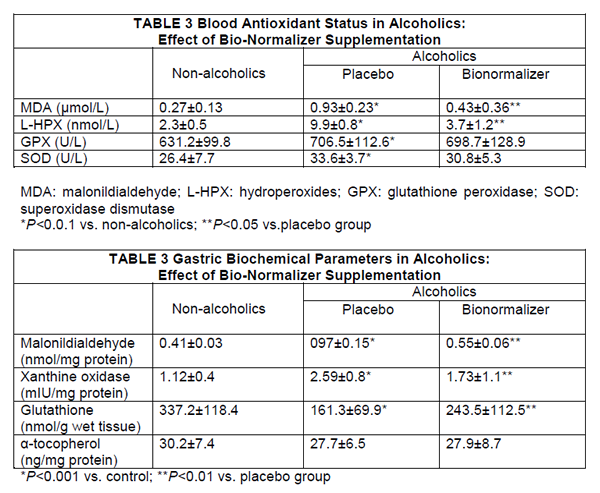
| TABLE 1 Dietary Intake in Alcoholic Population |
| Total energy (kcal) 2816±423 |
| Non-alcohol energy(kca1) 1879±253 |
| Protein (%) 14.4±2.7 |
| Carbohydrate (%)* 58.7±8.7 |
| Fat (%) 26.5±5.3 |
| Vitamin A (μg) ** 1512±789 |
| Vitamin E (mg) 8.2±1.9 |
| Vitamin C (mg) 82.8±23.7 |
*percentage of non-alcohol energy
**μg equivalent retinol = retinol (g) + carotene (μg)/6
| TABLE 2 Chemical Analysis of Bionormalizer (100G) |
| Available carbohydrate: 90.7g |
| Moisture 8.9g |
| Protein 0.3g |
| Fat none |
| Ash 0.1g |
| Folic acid 2µg |
| Niacin 0.24mg |
| Amino acids |
| Arginine 16mg |
| Lysine 6mg |
| Histidine 5mg |
| Phenylalanine 11mg |
| Tyrosine 9mg |
| Leucine 18mg |
| Isoleucine 9mg |
| Methionine 5mg |
| Valine 13mg |
| Alanine 12mg |
| Glycine 11mg |
| Proline 8mg |
| Glutamic acid 37mg |
| Serine 11mg |
| Threonine 8mg |
| Aspartic acid 27mg |
| Tryptophan 2mg |
| Carotene, cobalamin, thiamin, Riboflavin, total ascorbic acid, tocopherols, selenium: not detectedJapan Food Research Laboratories. Report n. 397100396-007 Tokyo, December 16, 1997. |
In the alcoholic group, 2 1 patients were smokers and 8 of them heavy smokers (over 40 cigarettes/day) while 11 control subjects were smokers and 2 of them were heavy smokers (not statistically significant difference).
Study Design
Patients were divided in 2 groups comparable as for age, gender, drinking and smoking habits. After overnight fasting, all patients underwent baseline blood chemical evaluation as described below. Urine samples were collected for the Schilling test.
During upper gastrointestinal endoscopy, biopsy samples of adequate size were taken each from the antrum and the gastric body. These serve the purpose of assessing routine histology, malonyldialdehyde (MDA) level, vitamin E and glutathione concentration and for assay vitamin B12 binding by intrinsic factor (IF). Further, gastric juice samples were collected and stored for assaying intrinsic factor and xanthine oxidase concentration. Endoscopic and biochemical assessments were repeated after 1 week of supplementation with Bionormalizer (Composition showed in Table 2, Osato Research Foundation, Gifu, Japan) 9g/nocte. During such period patients continued in their dietary habits and, in particular, with unchanged alcohol consumption, as ascertained later on.
Biochemical Methodology
Plasma biochemical measurement: Antioxidant status: Blood samples were taken using EDTA and anticlotting agent and immediately refrigerated in ice. The plasma was separated by centrifugation at 4°C. Plasma malonyldialdehyde as assayed by the method of Yagi (5) was modified by adding 0.01% butylated-hydrotoluen to the coloring solution to avoid the generation of thiobarbituric reactive substances (TBARS) reactive molecules during the procedure. The system was heated at 100°C for 45min. After cooling, the chromophore was extracted with 2mL of N-butanol by vigorous shaking and dried under constant nitrogen flux. The final powder was resuspended in 100μL of chromatographic solvent A and added to a high performance liquid chromatography HPLC) system (solvent A: 10mmol hexate sulfonic acid and H3PO4 adjusted to pH 3; solvent B; methanol). Tetra-ethoxy-propane served as a standard source of malonyldialdehyde which is a TBARS. All samples were processed in duplicate. Glutathione peroxidase and superoxide dismutase (SOD) activities of plasma were determined in a centrifugal analyser (Hoffman La Roche, Switzerland). Plasma lipid hydroperoxides, mostly phospholipid hydroperoxides, were assessed by hemoglobin catalyzed oxidation of 10-N-methylcarmoyl-3, 7-diemthylamino-10-H-phenothiazine after treatment with phospholipase D. Cumene hydroperoxide was used as standard. Plasma phospholipid was evaluated by a commercial available enzymatic kit (Boehringer Biochemia, Mannheim, Germany).
Gastric mucosal biochemical parameter: Malonyldialdehyde concentration in the gastric mucosa was determined as outlined above. Xanthine oxidase activity was assessed by applying to tissue the methodology cited below for gastric juice. For alphatocopherol determination pre-weighed biopsies were removed from the freezer and kept in ice to then be quickly blotted and weighed. Each biopsy sample was placed in a 5-mL tube with traces of butylated hydroxytoluene crystals and 20μL of a freshly made collagenase solution (20g/L containing 5mmol CaCl2/L). The tubes were incubated in a water bath at 45°C for 15min. On removal from the bath, 20µL of a freshly made pronase E solution (30g/L in 0.02mL phosphate buffer/L., pH 7.4) was added to each tube which was placed in ice. Each softened biopsy mixed with butylated hydroxytoluene crystals and enzymes was transferred to a glass microhomogenizer for 90s. The homogenate was then returned to its original tube. The homogenizer was washed twice by adding either 40μL of 5mmol CaC12/L and 40μL of water. The tubes were finally incubated in a water bath at 45°C for 15min. Soon afterwards, a quick extraction of α-tocopherol was carried out as follows. A freshly prepared solution (0.12mL) of 95% ethanol containing 800mg ascorbic acid/L, 0.55g sodium dodecyl sulphate/L and 125mg tocopherol acetate/L was added to each tube. The solution was immediately vortexmixed for 4s. Tubes were then placed in a multivortexer for 2min, centrifuged at 200 x g for 3m in at 20°C and the upper hexane layer was transferred to a
clean 5-mL tube. The residue was extracted a second time with a further 0.7mL hexane and shaken for 30s. The tubes were centrifuged and the supernatant added to the first hexane extract. The solvent was blown off by a stream of nitrogen at 400 C. the residue was reconstituted in 60μL ethanol and transferred to 0.3-mL autosampler vials which were stored at -70°C: the following day the specimens were injected by using the same mobile phase. To assay glutathione concentration, tissue samples were homogenized in
EDTA 0.02M at 4°C in the presence of 10μL of 50mM monobromobimane in acetonitrile and 20μL of 50mM N-ethylmorpholine at pH 8.4 for the derivatization of the sulphydryl groups. After 15min reaction in the dark, protein precipitation was obtained by 10% sulphosalicylic acid and final removal by centrifugation at 13.800rpm for 5min. The supernatant was filtered through a 0.45-μm Millipore and injected into a HPLC system. Excitation and emission wavelength were 370nm and 485nm,respectively. As derivatization standard, 10-100nmol of glutathione were injected into HPLC system prior to each sample processing.
Assay of intrinsic factor and xanthine oxidase in the gastric juice: For the intrinsic factor determination, gastric juice aliquots were depepsinized by titration to pH 11 using 10N sodium hydroxide, allowing the mixture to stand over ice for 15min and then titrating to pH7 with glacial acetic acid before storage at -20°C. Intrinsic factor concentration in gastric juice was determined by applying slight modifications to the methods described by Begley and al (6). Briefly, the standard curve was Chemical Company, USA) 0-10 units (made up to 100μL with distilled water), to 0.01N Tri HCl buffer (0.5mL, pH 7.4). Cobinamide, 0.25μL was added and the tubes incubated for 30min at 37°C. This was followed by 20μL of 57Co-labeled cyanocobalamin (Amersham Corporation, Illinois, USA) per tube and the mixture incubated for a further 30min a 37°C. Free (57CO) cyanocobalamin was separated from IF-bound (57CO) cyanocobalamin, by the addition of 1mL/tube of albumin-coated charcoal. The standard curve was prepared by plotting increasing concentrations of IF against B/(B+F),where Bi s the radioactivity in the supernatant (IF -bound) and F the radioactivity in the charcoal. IF in gastric juice was assayed using 100μL of gastric juice treated as above and the concentrations determined from the standard curve. For xanthine oxidase determination, gastric juice samples were checked for pH value by a digital pH meter and centrifuged at 300rpm for 5min to obtain a clear supernatant. Supernatants were extracted by using a 3:5v/v chloroform-ethanol mixture and then centrifuged at 10000rpm for 15min. The clear upper layer thus obtained was used for xanthine oxidase activity determination, as described by Prajda et al (7). Gastric juice pH was checked by a pH meter as well.
Schilling test: Urine samples were used for the established dual-isotope technique (DIST), following strict methodological quality control as advised by a very recent validation study (8). Mean values were expressed as: Free 57Co-Vitamin B12 excretion (%) and IF-Bound 57Co-Vitamin B12 excretion (%).
Assay of vitamin B12 binding by IF: Pooled gastric mucosal biopsy samples from the body were immediately placed in Tris HCl, pH 10.0, homogenized in 7.5w/v Tris buffer and centrifuged at 14.000rpm for 20min at 4°C. Supernatants were diluted with saline 1:300 for IF/vitamin B12 binding assay. This was assayed following incubation of concentrated 0.5U/mL IF with reaction mixture and the solutions were diluted to 0.05 units/mL of IF for assay. For gastric homogenate incubation, a dilution of gastric samples of 1:7.5w/v was used for incubations and a final dilution of 1:300 of gastric homogenates were used for assay. Dilutes IF of gastric homogenates were added to 50pg of cyanocobalamin containing 0.025μCi of 57Co-cyanocobalamin. The number of counts remaining in solution after precipitation with hemoglobin-coated charcoal were determined. The specificity of the gastric homogenate IF for B12 was ascertained by incubating anti-IF antibodies stored from the serum of patients with pernicious anemia and using normal serum as control.
Statistical analysis: Data were analyzed using the non-parametric Mann-Whitney U test and correlations were tested using Kendall-Tau test for non-parametric data.
RESULTS
Serum Biochemical Assays
Routine blood chemistrγ did not change, during the study period, irrespective of the treatment employed. As expected, plasma MDA level and lipid hydroperoxides concentration in chronic alcohol abusers were significantly higher than in controls (P<0.001) (Table 3). However, despite unchanged alcohol consumption, 1-week supplementation with Bionormalizer brought about a significant decrease of both parameters (P<0.05),although they did not entirely revert to mean control value. Either glutathione peroxide and superoxide dismutase were significantly higher than in non-alcoholic patients (P<0.05) and remained unchanged after Bionormalizer supplementation.
Gastric Mucosal Histology and Biochemical Assays
Routine histology showed mild-to-moderate degree of superficial chronic gastritis without Helicobacter pylori infection. No case of gastric atrophy was recorded. After 1-week supplementation with either placebo or Bionormalizer, non-significant change occurred. Either malonyldialdehyde and xanthine oxidase concentration in the gastric mucosa in alcoholics were significantly higher than in nonalcoholics (P<0.001) (Table 4). After 1 week of Bionormalizer supplementation such parameters significantly decreased within normal level (P<0.05). Gastric mucosal glutathione was markedly depressed in alcoholics (P<0.001) and partly recovered after Bionormalizer supplementation (P<0.05 vs. placebo). On the contrary, vitamin E level in alcoholics was comparable to that of the teetotaler group and remained unaltered whatever the treatment employed.
Intrinsic Factor and Xanthine Oxidase Concentration in the Gastric Juice
As shown in Figure 1, the concentration of intrinsic factor in the gastric juice was within normal limits either in controls and in alcoholics and was not affected by Bionormalizer treatment. Xanthine oxidase activity in gastric juice of alcoholics was significantly higher compared with the control group (P<0.01). Bionormalizer treatment seemed to bring about a trend decrease of xanthine oxidase concentration but this did not reach a statistical significance. Gastric pH was within normal limit in controls and alcoholic patients either at the entry into the study and at the end.
Dual-isotope Schilling Test
Results are presented in Figure 2. At the entry, as compared to controls whose single values fell within normal range, 7 alcoholic patients had very low free 58co-vitamin B12 excretion percentage (below 10%) and abnormally high bound/free ratio (about 2) although this did not determine a statistically significant difference in the group as a whole. However, after 1 week of Bionormalizer supplementation each single alcoholic patient showed normal values. In particular, when using a cut-off level of 10% and thus taking separately such a subgroup of alcoholics, Bionormalizer supplementation enabled a statistically significant improvement (P<0.05)with values comparable to controls and with normalization of the bound/free ratio
Vitamin B12 Binding by IF
As compared to control, gastric mucosa homogenates obtained from alcoholics showed a significantly reduced intrinsic factor binding of vitamin B12 (P<0.001) (Figure 3). Bionormalizer supplementation for 1 week reverted to normal this abnormality (P<0.01 vs. placebo).
DISCUSSION
Cobalamin is a water-soluble vitamin that cannot be synthesized by mammalian species. Therefore, man is entirely dependent on dietary sources of cobalamin whose average daily intake is theoretically 2-4-fold the physiological requirement. Its absorption relies upon an orderly and multifaceted sequence of events which can be briefly summarized in a gastric stage, intestinal transport and uptake of intrinsic factor-cobalamin complex by ileal mucosa and cobalamin transcytosis. Binding to intrinsic factor which is present in most gastric cell types in the human stomach and whose secretion is considerably greater than the human daily requirement is the crucial event in cobalamin absorption. Shaw et al (3) has shown on experimental ground that such binding is markedly inhibited by a free radicals-generating system such as a medium of
Acetaldehyde and xanthine oxidase, thus providing a stimulating background for clinical studies in alcoholism. Indeed, more recently it has been suggested that the increased free radicals production observed in the presence of ethanol could be related to the oxidation through xanthine oxidase of locally produced acetaldehyde (9). However, to the best of our knowledge, the issue of possible interference of ethanol consumption and following free radicals generation in cobalamin absorption has not been further addressed. Indeed, despite some conflicting reports, the involvement of oxygen radicals in ethanol induced gastric injury has been extensively confirmed in “in vitro” as well as “in vivo” studies (10, 11). Accordingly, in order to study a possible therapeutic intervention with antioxidants on a presumptive ethanol-induced cobalamin absorption abnormality, in the present investigation we used Bionormalizer which is a novel food supplement prepared by yeast fermentation of medicinal plants and is heat-and acid resistant (12). Although it is devoid of detectable amounts of antioxidative vitamins or enzymes (Table 2), this product has been demonstrated to scavenge hydroxyl radicals in cell-free systems (13, 14) and, more recently such properties have been confirmed in clinical studies as well (4,15,16). Despite unchanged drinking habits, 1-week Bionomalizer supplementation brought about a significant decrease of free radicals-related parameters either in the blood (MDA, xanthine oxidase, glutathione). The mucosa of the stomach and of the upper gastrointestinal tract is particularly rich in xanthine oxidase, the rate-limiting enzyme of purine catabolism, which generates superoxide anion as a by-product. Further, his enzyme might show an enhanced activity at the upper gastrointestinal mucosa location under a number of local injuries with a following burst of local free radicals generation. lndeed, there is experimental evidence showing a protecting role of xanthine oxidase inhibitors on ethanol-induced gastric mucosa damage (11). When investigating in our study any possible ethanol-related cobalamin absorption abnormality, we preferred not to base it on “on spot” plasma cobalamin evaluations. lndeed, the unreliability of such determination has been critically pointed out in up-to date reviews (17) and very recently low levels have been observed in a high percentage of nonmetabolically cobalamin-deficient individuals (18). Further, we have purposely ruled out subjects or patients with Helicobacter pylori infection, due to its recently shown interference with food-cobalamin absorption (19). Interestingly, athough the alcoholics showed a normal intrinsic factor secretion in the gastric juice, they exhibited a markedly depressed intrinsic factor-cobalamin binding in the “ex vivo” study. Moreover, nearly 23% of them had an abnormal Schilling test which is considerably higher than the 5% incidence of low serum vitamin B12 levels in alcoholics, as reported in the literature (2). Both these impairments reverted to normal after Bionormalizer supplementation. Following the experimental study of Shaw et al (3), one might postulate that the antioxidative action played by Bionormalizer, with significant decrease of gastric mucosal concentration of malonyldialdehyde and xanthine oxidase activity together with a partial restoration of glutathione, has enabled a recoverγ of normal cobalamin absorption.
Our alcoholic population showed higher levels of xanthine oxidase concentration also in the gastric juice and Bionormalizer supplementation caused only a not-significant trend decrease. Nonetheless, it has to be considered that the mechanism of xanthine oxidase secretion pathways into the gastric lumen is not fully elucidated. Further, our alcoholics, whose depleted blood antioxidant status only partly improved in the Bionormalizer group, continued to heavily consume alcohol during the study and it has been well documented that plasma level of xanthine oxidase activity increases up to 10-fold in active alcoholics as a likely response to hepatic cell inury (9). While atrophic gastritis or intestinal pathology have been until recently implicated to as relevant causes of vitamin B12 deficiency in the elderly population (20), this cannot be reconciled with the histological and clinical findings in our alcoholic population. The group of Loguercio et al (21) has recently confirmed the clinically relevant importance of glutathione replenishment in preventing ethanol-induced acute gastric damage. Although the inner mechanism of action of Bionormalizer is still the issue of ongoing studies at several research centers, the biologically-active availability of Bionormalizer substrates for glutathione synthesis such as glutamic acid, glycine and methionine as well as its possible effect on local oxidative burst from neutrophil, as experimentally demonstrated (22), hypotheses worth considering. Finally, Korkina (23) has shown a significant chelating action of Bionormalizer in experimental hemochromatosis. This last finding is of interest in keeping with the knowledge that iron plays a role in promoting free radical-induced lipid peroxidation and mediating gastric cell damage (24). Indeed, in the study of Shaw et al (3) the addition of ferritin to the acetaldehyde-xanthine oxidase medium further impaired the intrinsic factor-vitamin B12 binding.
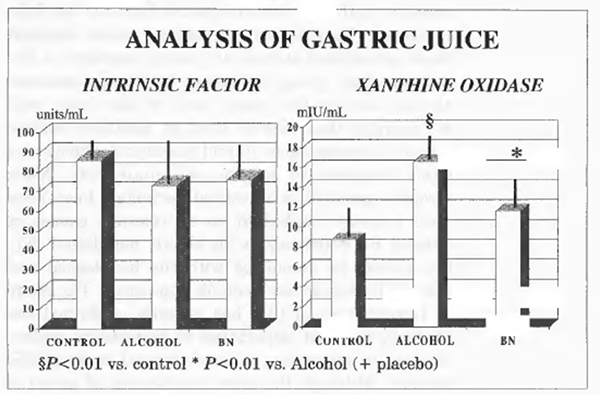 FIGURE 1 Analysis of gastric juice.
FIGURE 1 Analysis of gastric juice.
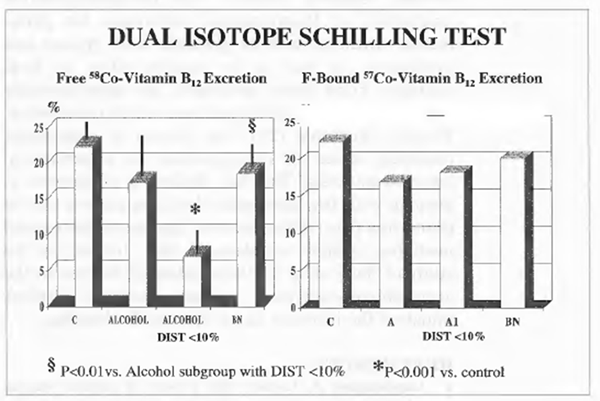 FIGURE 2 Dual-isotope schilling test.
FIGURE 2 Dual-isotope schilling test.
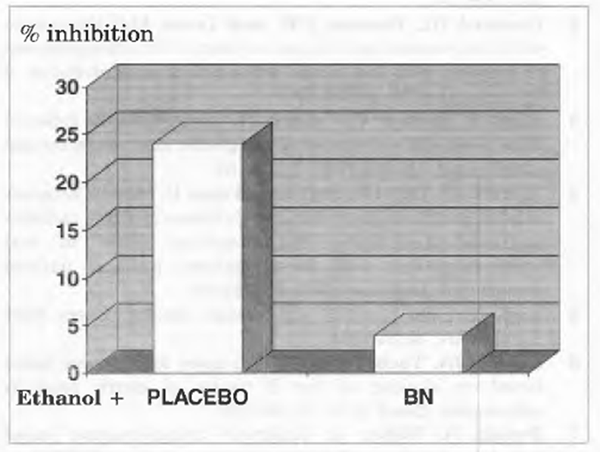 FIGURE 3 Vitamin B12 binding by IF, in vitro incubation of IF with 57Colabelled cyanocobalamin.
FIGURE 3 Vitamin B12 binding by IF, in vitro incubation of IF with 57Colabelled cyanocobalamin.