| Title | EFFICACY OF BIO-CATALYZER α.ρ No. 11 (BIO-NORMALIZER) SUPPLEMENTATION AGAINST PEROXYL RADICAL-INDUCED OXIDATIVE DAMAGE IN RAT ORGAN HOMOGENATES. |
|---|---|
| Year | 1996 |
| Author | Lucila Marcocci1,2, Rosella D’ Anna1, Liang-Jun Yan1, Nobuya Haramaki1 and Lester Packer1. |
| Publisher | Biochemistry and Molecular Biology International |
EFFICACY OF BIO-CATALYZER a.r No. 11 (BIO-NORMALIZER) SUPPLEMENTATION AGAINST PEROXYL RADICAL-INDUCED OXIDATIVE DAMAGE IN RAT ORGAN HOMOGENATES.
Lucila Marcocci1,2, Rosella D’ Anna1, Liang-Jun Yan1,
Nobuya Haramaki1 and Lester Packer1.
1Membrane Bioenergetics Group, Department of Molecular and Cell Biology,
University of California, Berkeley, CA 94720-3200. 2Dipartimento Scienze
Biochimiche “A. Rossi Fanelli” Universita’ di Romaa “La Sapienza” Piazzale
Aldo Moro 5, 00185 Roma, Italy.
Received December 18, 1995
To better delineate the antioxidant potential of Bio-Catalyzer a.r No. 11 (Bio-Normalizer), a natural food supplement proposed as an antioxidant agent, we investigated the efficacy of Bio-Normalizer supplementation to protect rat organ homogenates against oxidative damage induced in vitro by peroxyl radicals generated in the hydrophobic or in the hydrophilic phase. Bio-Normalizer supplementation efficiently protected rat kidney homogenates against the accumulation of thiobarbituric reactive substances (TBARS), the formation of protein carbonyl derivatives and the depletion of a-tocopherol induced by peroxyl radicals generated from the hydrophobic azo-initiator 2,2’-azobis (2,4-dimethylvaleronitrile) (AMVN). It also protected the heart but not the liver or the brain homogenates. Bio-Normalizer supplementation did not have effect in any organ homogenates when peroxyl radicals were generated from the hydrophilic azo-initiator 2,2’-azobis (2-amidinopropane dihydrochloride) (AAPH). In vitro direct addition of aqueous solutions of Bio-Normalizer to the organ homogenates was ineffective against AMVN or AAPH-induced oxidative damage. Our findings expand previous reports on the antioxidant activity of Bio-Normalizer. They confirm that supplemented Bio-Normalizer protects against peroxyl radical-induced oxidative damage and suggest that its antioxidant action depends on in vivo bioactivation, it is organ specific and it is limited to damage induced by peroxyl radicals generated in the hydrophobic phase.
INTRODUCTION
Bio-Catalyzer a.r No. 11 (Bio-Normalizer), a food supplement prepared from a yeast fermented mixture of various plants (i.e. Carica papaya Linn, Pennisetum pupureum Schum., Sechium edule Swartz), has been recently proposed as an antioxidant agent. It has been shown to scavenge hydroxyl radical in vitro (1,2), and to protect rodents against oxidative damage induced in the brain by aging (3), by iron-treatment (4) or by ischemia-reperfusion injury (5). Furthermore, a study conducted in our laboratory indicated that Bio-Normalizer supplementation protects isolated rat hearts against ischemia-reperfusion injury, and rat heart homogenates against AMVN [(2,2’-azobis (2,4-dimethylvaleronitrile)]-induced oxidative damage (6).
To further define the antioxidant potential of Bio-Normalizer, in the study reported here, we expanded our previous analysis on the efficacy against oxidative damage induced by in vitro generated peroxyl radicals. We thus tested the effect Bio-Normalizer supplementation to protect various rat organ homogenates, in particular kidney, heart, liver and brain against oxidative damage induced by peroxyl radicals generated in the hydrophobic or in the hydrophilic phase from the azo-initiators AMVN (2,2’-azobis (2,4-dimethyl valeronitrile) or AAPH (2,2’-azobis (2-amidinopropane) dihydrochloride), respectively (7). To better clarify the molecular mechanism of Bio-Normalizer directly added to organ homogenates.
Evidence were obtained that Bio-Normalizer supplementation protects rat organ homogenates against peroxyl radicals induced oxidative stress. However, the antioxidant action of supplemented Bio-Normalizer appeared to be specific for the organs and the site of peroxyl radicals generation as well as it seems to depend on the in vivo bioactivation.
MATERIALS AND METHODS
Chemicals: Bio-Normalizer was a kind gift of the Osato Research Institute, Gifu, Japan. AMVN and AAPH were purchased from Polysciences, Inc. (Warrington, PA), and were used as ethanol or aqueous solutions, respectively. Deferoxamine mesylate, bovine serum albumin, ethylendiamine tetraacetic acid (EDTA), and butylated hydroxytoluene (BHT) were purchased from Sigma Chemical Co. (St. Louis, MO). All other chemicals were of analytical grade.
Animals: Male Sprague-Dawley rats (250-300 g) were obtained from Bantin and Kingman (Fremont, CA). All animals were allowed ad libitum access to water with or without 0.1% (w/v) Bio-Normalizer and fed rat chow (Harlan Teklad Rodent Diet, Madison, WI) for 6 weeks.
Peroxyl radical-induced oxidative stress: Rat organs, isolated from animals supplemented with or without Bio-Normalizer, were homogenized at concentration of 200 mg tissue/ml in nitrogen gassed 50 mM phosphate buffer (pH 7.4) containing deferoxamine mesylate (1 mM, final concentration) by using a Ultraturrax homogenizer; they were then sonicated for 90 seconds using a tip sonicator (Branson Sonic Power Co.) and incubated at a concentration of 10 mg protein/ml with or without AMVN or AAPH (5 mM final concentration) at 42°C up to 2.5 hrs. In some experiments Bio-Normalizer was added as aqueous solution to tissue homogenates prepared from control animals and the samples were incubated with or without the azo-initiators under the conditions described above. Protein concentration of the homogenates was measured by Lowry assay (8) with bovine serum albumin as standard.
TBARS assay: After incubation, BHT (200 mM, final concentration) was added to the samples to stop the oxidative reaction. TBARS formation was immediately analyzed as described by Slater (9) using 1,1,3,3-tetramethoxypropane (TMP) as standard.
Protein carbonyl assay: After incubation, tissue homogenates were mixed with EDTA (100 mM, final concentration) and stored at -80°C until the assay. Protein carbonyl derivatives were then measured as previously reported (10).
Tocopherol assay. After incubation, tissue homogenates were mixed with BHT (4.5 mM, final concentration) and stored at -80°C until the assay. The tissue a-tocopherol content was then measured by HPLC as reported by Podda et al. (11).
Statistical Analysis: All data are expressed as mean ± S.E.M. Comparisons were carried out using the Student-t test. A difference was considered to be significant when p<0.05.
RESULTS
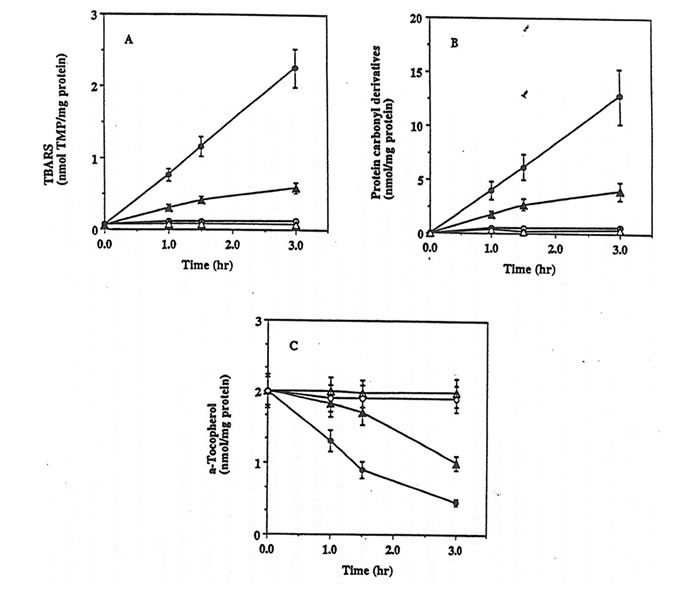
The exposure of rat kidney homogenates in PBS at 42°C to peroxyl radicals generated from the thermal decomposition of the hydrophobic azo-initiator AMVN resulted in a significant oxidative damage as documented by the time-dependent accumulation of TBARS, the formation of protein carbonyl derivatives, and the depletion of a-tocopherol (Fig. 1, parts A-C closed circles). A statistically significant lower level of AMVN-dependent oxidation of lipids and proteins as well as depletion of a-tocopherol was measured in kidney homogenates isolated from rats supplemented for 6 weeks with Bio-Normalizer than from control animals (Fig. 1, parts A-C closed triangles).
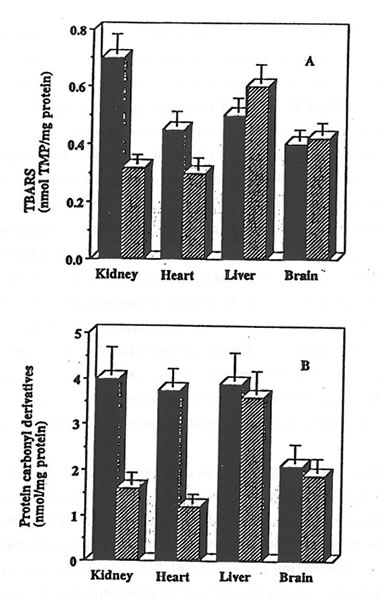
The exposure of rat heart, liver and brain homogenates to AMVN also resulted in a consistent damage to the tissue lipids and proteins. Bio-Normalizer supplementation effectively protected rat heart homogenates against oxidative damage, thus confirming previous data (6). However, it did not protect liver or brain homogenates: similar rates of TBARS formation and of protein carbonyl derivative accumulation were measured in the samples prepared from control or from Bio-Normalizer supplemented animals (Fig. 2, parts A and B).
To better define the molecular mechanism of Bio-Normalizer action, we analyzed the efficacy of Bio-Normalizer supplementation against damage induced by peroxyl radicals generated from the hydrophylic azo-initiator AAPH. Bio-Normalizer supplementation did not protect any organ homogenates against AAPH-induced oxidative stress. In fact, similar rates of AAPH-dependent TBARS or protein carbonyl derivative formation were observed in kidney, heart, liver and brain homogenates prepared from control or from Bio-Normalizer supplemented rates (data not shown).
The antioxidant activity of solutions of Bio-Normalizer directly added to rat organ homogenates was also tested. Bio-Normalizer (0.200 or 1 mg/ml, final concentration) added to kidney, heart, liver or brain homogenates prepared from control animals did not prevent the AMVN or AAPH-dependent accumulation of TBARS or protein carbonyl derivative formations (data not shown).
Discussion
The assessment of the efficacy of a compound to protect against peroxyl radical-induced oxidative damage is an important test to define antioxidant properties. Peroxyl radicals, formed in solution or in the lipophilic phase by the reaction of carbon-centered radicals with oxygen, are biologically relevant oxygen species for the damage to cellular constituents. Their formation is the major chain-propagating step during lipid peroxidation, and they have been shown to inactivate soluble enzymes (12).
Antioxidant efficacy of Bio-Normalizer supplementation against oxidative damage induced in vitro by peroxyl radicals generated in the hydrophobic phase from the azo-initiator AMVN has been documented in rat heart homogenates (6). In the paper reported here, to further characterize the antioxidant potential of Bio-Normalizer and thus strengthen the suggestions for a use of this natural product as an antioxidant agent, we verified the effect of Bio-Normalizer supplementation against peroxyl-radical-induced damage in the other rat organ homogenates.
Bio-Normalizer orally administered effectively protected rat heart and kidney homogenates against peroxyl radical-induced oxidative stress; however, its action depended on the site of radical generation. In fact, Bio-Normalizer administration protected heart or kidney homogenates against oxidative damage induced by peroxyl radicals generated in the hydrophobic phase from the azo-initiator AMVN but not against oxidative damage induced by peroxyl
Figure 1. Effect of Bio-Normalizer supplementation against AMVN-induced oxidative damage in rat kidney homogenates.
Rat Kidney homogenates (10 mg/protein/ml) prepared from control (circles) or from Bio-Normalizer supplemented animals (triangles), were incubated in PBS at 42°C with (closed symbols) or without (open symbols) 5 mM AMVN. The content of TBARS (part A), protein carbonyl derivatives (part B) and a-tocopherol (part C) were then assayed. Mean ± S.E.M. of 3 different samples.
Figure 2. Effect of Bio-Normalizer supplementation on AMVN-induced accumulation of TBARS and protein carbonyl derivatives in various rat organs homogenates.
Rat tissue homogenates (10 mg protein/ml) prepared from control (solid bars) or from Bio-Normalizer supplemented animals (hatched bars) were incubated in PBS at 42°C for 1 hr with 5 mM AMVN. TBARS (part A) and protein carbonyl derivatives (part B) were measured and given as mean ± S.E.M. of 6 different samples.
radicals generated in the hydrophilic phase from the azo-initiator AAPH. As in biological systems the accessibility of a radical to its scavenger can be an important factor in limiting scavenging action, we suggest that upon supplementation the kidney and heart tissues become enriched with a hydrophobic component of Bio-Normalizer mixture or one able to interact with hydrophobic domains. Alternatively, hydrophobic metabolites of Bio-Normalizer, generated upon in vivo bioactivation, could be responsible for the observed protection.
We did not observe protection either in the liver or in the brain homogenates against oxidative damage induced by AMVN or by AAPH. We suggest that in these organs Bio-Normalizer components are metabolized less effectively than in the heart or in the kidney to the antioxidant active forms; also, we do not exclude that in these organs peroxyl radical-scavenging component of Bio-Normalizer maya reach low concentrations. In particular, we can not rule out the possibility that Bio-Normalizer components able to protect against peroxyl radical-induced damage do not cross the blood-brain barrier.
Bio-Normalizer supplementation has been documented to protect the rat brain against lipid peroxidation induced by iron treatment or by aging (3, 4). Although our data do not exclude that Bio-Normalizer supplementation can act in the brain by chelating metals, by scavenging hydroxyl radical or by changing the cellular pattern of antioxidant enzymes as it has been proposed (3, 4), they suggest that the protective effect is not directly due to peroxyl radical-scavenging activity.
Aqueous solutions of Bio-Normalizer directly added to tissue homogenates prepared from control animals did not protect against oxidative damage induced by AMVN or by AAPH. These data support the idea that in vivo bioactivation may be required to transform Bio-Normalizer into an active compound against damage induced by peroxyl radicals generated in hydrophobic phase and they indicate that in our experimental system Bio-Normalizer does not protect against oxidative damage induced by peroxyl radicals generated in hydrophilic domains.
In conclusion, Bio-Normalizer supplementation provided rat heart and kidney tissues with antioxidant defence against oxidative damage induced by peroxyl radicals generated in hydrophobic phase. Our data strengthen the evidence for the antioxidant action of this natural product and propose it as a possible agent to protect some organs against oxidative damage-dependent pathological conditions.
REFERENCES
- Santiago, L.A., Osato, J.A., Hiramatsu, M., Edamatsu, R. and Mori, A. (1991) Free Rad. Biol. Med. 11, 379-383.
- Santiago, L.A., Osato, J.A. and Mori, A. (1992) Med. Sci. Res. 20, 27-28.
- Santiago, L., Osato, J. A. and Mori, A. (1993) Neurochem. Res. 18, 711-717.
- Santiago, L.A., Osato, J.A., Kabuto, H. and Mori, A. (1992) Med. Sci. Res. 21, 139-141.
- Santiago, L.A., Osato, J.A., Ogawa, N. and Mori, A. (1993) NeuroReport 4, 1031-1034.
- Haramaki, N., Marcocci, L., D’Anna, R., Yan, L.-J., Kobuchi, H. and Packer, L. (1995) Biochem. Mol. Biol. Int. 6, 1263-1268.
- Niki, E. (1990) Meth. Enzymol. 186, 100-108.
- Lowry, O.H., Rosenbrough, H.J., Farr, A.L. and Randall, R.J. (1951) J. Biol. Chem. 193, 265-275.
- Slater, T.F. (1984) Meth. Enzymol. 105, 283-293.
- Reznick, A.Z. and Packer, L. (1994) Meth. Enzymol. 233, 357-363.
- Podda, M., Tritschler, H.J., Ulrich, H. and Packer, L. (1994) Biochem. Biophys. Res. Commun. 204, 98-104.
- Haliwell, B. and Gutteridge, J.M.C.(1989) Free Radials in Biology and Medicine, Oxford University Press, Oxford.