| Title | ANTIMICROBIAL POTENTIAL OF BIO-CATALYZER α.ρ NO. 11 (BIO-NORMALIZER) AGAINST ENTERIC MICROORGANISMS |
|---|---|
| Year | 1994 |
| Author | James Akira Osato, Gloria de Castro-Bernas, Gemma M. Remo, Milagrosa S. Cuadra, Godofredo Urbano, Ma. Russell B. Abrigo, Librado A. Santiago, and Kazuhiro Takamizawa |
| Publisher | Acta Manilana |
Antimicrobial Potential of Bio-catalyzer α.ρ No. 11 (Bio-normalizer) Against Enteric Microorganisms
James Akira Osato 1,2, Gloria de Castro-Bernas3, Gemma M. Remo4, Milagrosa S. Cuadra2, Godofredo Urbano4, Ma. Russell B. Abrigo4, Librado A. Santiago2,5, and Kazuhiro Takamizawa1
1The United Graduate School of Agricultural Science, Gifu University, 1-1 Yanagido, Gifu 500, Japan
2Osato Research Foundation, Inc., 12-Minami machi, Bairin, Gifu 500, Japan
3Research Center for the Natural Sciences, University of Santo Tomas, Manila 1008, Philippines
4Osato Research Foundation, Inc., 12th Floor, Royal Match Building, 6780 Ayala Avenue, Makati, Metro Manila, Philippines
5Department of Neuroscience, Institute of Molecular and Cellular Medicine, Okayama University Medical School, 2-5-1 Shikata-cho, Okayama 700, Japan
Abstract. A total of 141 bacterial isolates were obtained and identified from 84 gastric and rectal exudates of patients suffering from gastric ulcer, enterocolitis, diarrhea, gastroenteritis, internal and external hemorrhoids. The clinical isolates identified were Enterobacter aerogenes, Escherichia coli, Salmonella typhi, Citrobacter freundii, Pseudomonas aeruginosa, Enterobacter sakazakii, Edwardsiella tarda, Proteus vulgaris and Enterobacter agglomerans.
The minimum inhibitory concentration of Bio-catalyzer a.r No.11 (Bio-normalizer), a health food supplement made by yeast fermentation of Carica papaya Linn. and traditional Japanese herbs, was found out to be 500 mg/ml. Its antimicrobial activity against these clinical isolates was determined by paper disc diffusion method.
Bio-normalizer showed thnning of growth on 89% of the isolates. The results suggest that Bio-normalizer has the potential to stop the growth of the most commonly encountered enteropathogenic and opportunistic microorganisms which may abound during abnormal conditions in the gastrointestinal tract.
Keywords: antimicrobial, Bio-normalizer, enteric microorganisms
INTRODUCTION
Over four thousand antimicrobial agents have been isolated from natural sources and
more than thirty thousand compounds are acquired from natural antibiotics. A number of these compounds are used efficiently in the field of infectious diseases[1]. But recently, residual toxicity and side effects as well as cross-resistance of infections had been manifested, thus, upholding the quest for clinically applicable natural antimicrobials.
Bio-catalyzer a.r No.11 (Bio-normalizer) is a natural health product made by yeast
fermentation of Carica papaya Linn.and other tropical herbs with glucose as the main carbon source [2]. It is commercially sold in Japan and in the Philippines as a food supplement. Bio-normalizer is a potent and stable hydroxyl radical scavenger [2,3]. It has the ability to increase gamma interferon in the body suggesting that it can enhance the activity of lymphocytes and helper T-cells in coping with the infection and diseases [4]. Moreover, it can regulate superoxide production as well as stimulate white blood cell and macrophage activity.
Its major component, Carica papaya Linn. which is a known medicinal herb, is used as
antihelminthic, stomachic, diuretic, laxative, remedy for skin problems, among others[5]. It is bacteriostatic to most commonly encountered microorganisms [6] and a scavenger of free radicals [6,7].
Administration of Bio-normalizer was observed to improve a wide range of medical cases
from simple colds to chronic diseases such as cardiovascular disorders, brain dysfunctions, gastrointestinal diseases, among others. The present study was undertaken to look into Bio-normalizer’s effect on a number of opportunistic and pathogenic orgasnisms isolated from gastrointestinal specimens.
EXPERIMENTAL
Test sample. Bio-normalizer was supplied by Sun-O International, Inc., Gifu, Japan.
Test Organisms. Standard test organisms such as Escherichia coli, Enterobacter cloacae,
Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus subtilis and Proteus vulgaris used in the determination of the minimum inhibitory concentration (MIC) were originally obtained from the American Type Culture Collection and preserved by the Microbiology Section of the University of Santo Tomas Research Center for the Natural Sciences (USTRCNS) (Manila, Philippines). The clinical isolates subjected to antimicrobial assay such as E. aerogenes, E. coli, Salmonella typhi, Citrobacter freundii, P. aeruginosa, E. sakazakii, Edwardsiella tarda, P. vulgaris and E. agglomerans were identified from specimens of patients with gastrointestinal disorders.
Clinical Specimens. A total of 84 gastric and rectal exudates were aseptically obtained
through endoscopy (visualization and inspection of the interior portion of a hollow organ) and proctoscopy (dilation and inspection of the rectum) by the medical staff of the Gastroenterology Section of the Department of Medicine, UST Hospital (Manila, Philippines) from patients clinically diagnosed to be suffering from gastroenteritis, enterocolitis, diarrhea, gastric ulcer, internal and external hemorrhoids.
Isolation and Characterization. Gastric and rectal samples were streaked on to blood agar
and nutrient agar plates which were incubated for 24 hrs. at 37ºC to yield colonies. Incubation was extended to 48 hrs. when no growth was observed. Isolated colonies were characterized culturally, morphologically and biochemically[8]. Analytical Profile Index (API Systems, France) was used to confirm the identity of the isolates.
MIC Determination. Various concentrations of Bio-normalizer ranging from 100-1000
mg/ml were tested against the standard organisms and common clinical isolates using agar cup method and paper disc diffusion method [9,10]. Results showed no significant difference between the two methods used. Bacterial suspensions were normalized to contain 3 x 108 organisms/ml compairing with standards for turbidity measurements (Mc Farland Nephelometer Barium Sulfate Standards [9].
Antimicrobial Analysis. To determine the antimicrobial activity of Bio-normalizer using
its MIC, isolates were subjected to paper disc diffusion method [9,10]. Assay for each isolate was done in triplicates. The plates were incubated at 37ºC for 24 hrs. and zones of inhibition were measured in mm diameter.
RESULTS AND DISCUSSION
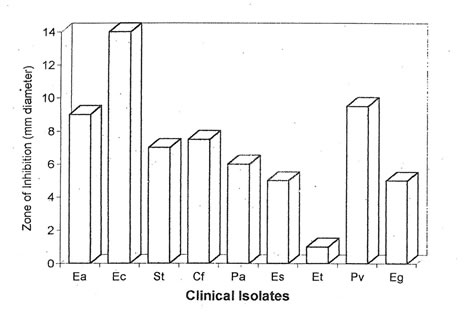
One hundred and forty-one isolates were characterized and identified from 84 specimens collected from patients with gastrointestinal disorders. The clinical isolates identified, in order of frequency, were E. aerogenes, E. coli, S. typhi, C. freundii, P. aeruginosa, E. sakazakii, E. tarda, P.vulgaris and E. agglomerans. The MIC of Bio-normalizer was found to be 500 mg/ml. A range of 5-14 mm thinning of bacterial growth was observed in 89% of the clinical isolates wherein E. coli has the largest zone of thinning (Fig. 1). It could be inferred that Bio-normalizer has the potential antimicrobial property since it has the capacity to stop the growth of the microorganisms.
Figure 1. Antimicrobial activity of Bio-normalizer (500 mg/ml) against 141 isolates from 84 clinical specimens of patients with enterocolitis, diarrhea, gastroenteritis, gastric ulcer, internal and external hemorrhoids. Ea – E. aerogenes; Ec – E. coli; St – Salmonella typhi; Cf – C. freundii; Pa – P. aeruginosa; Es – E. sakazakii; Et – E. tarda; Pv – P. vulgaris; Eg – E. agglomerans.
Our study showed that the predominant group of bacteria isolated in the gastrointestinal tract belong to Enterobacteriaceae. It includes both the pathogenic bacteria, which infect or parasitize normal individuals, and the opportunistic bacteria, which occur as a result of abnormalities in host defenses. Opportunistic E. aerogenes, P. vulgaris, E. tarda and C. freundii are responsible for outbreaks of diarrhea [11,12] while E. agglomerans, E. sakazakii and P. aeruginosa are most frequently implicated with human infections and gastroenteritis [13,14]. Salmonella species are some of the most incessantly identified enteric pathogens [15] involved in gastroenteritis [16,17], enterocolitis [16] and diarrhea [18]. Enteropathogenic E. coli infections, being a leading cause of diarrhea [11,15,18] most especially among infants in developing countries [20], are also associated with ulcerative colitis [21] due to toxins they produce. The ability of Bio-normalizer to stop the growth of the most common pathogens, may play a role in controlling the outburst of bacterial colonization, thereby, allowing the immune system to subdue the infection. It may not completely eliminate the infection, but it will prevent the microorgaisms from reproducing.
We, therefore, recommend further investigations to elucidate the mechanism of Bio-normalizer’s antimicrobial action.
ACKNOWLEDGEMENTS
The authors thank Dr. Jesus Perez (Section Head), Dr. Shirley Pua, Dr. Paez and the staff of the Gastroenterology Section, Department of Medicine, UST Hospital (Manila, Philippines) for providing the specimens from patients. Sincere thanks are in order for Mrs. Nimfa Chua of the Microbiology Section, USTRCNS for giving us the standard microorganisms in these experiments. We also thank Dr. Adelwisa Ortega of the Dept. of Microbiology, College of Public Health, University of the Philippines for her invaluable support and guidance.
REFERENCES
- T. Suzuki. Proc. Second Asia-Pacific Biotechnology Congress (1991) 154.
- L.A. Santiago, J. A. Osato and A.Mori. Free Radical Biol. Med. 11 (1991) 379.
- L.A. Santiago, J.A. Osato and A. Mori. Med. Sci. Res. 20 (1992) 27.
- L. A. Santiago, K. Uno, T. Kishida, F. Miyagawa, J. A. Osato and A. Mori. Neurosciences. 20s (1994) 149.
- E. Quisumbing. Medicinal Plants of the Philippines. Katha Pub., Quezon City, 1978.
- J. A. Osato, L.A. Santiago, G. M. Remo, M.S. Cuadra and A. Mori. Life Sciences 53 (1993) 1383.
- L.A. Santiago, J. A. Osato and A. Mori. Neurosciences. 18 (1992) 189.
- A.K. Raymundo, A. F. Zamora and I.F. Dalmacio. Manual on Microbiological Techniques. University of the Philippines, Technology and Livelihood Resource Center, 1991.
- O.C. Grove and W.A. Randall. Assay Methods of Antibiotics A Laboratory Manual. Medical Encyclopedia Inc., New York, 1995.
- F. Kavanagh. Analytical Microbiology Vol.II. Academic Press, Inc., New York, 1963
- W. A. Volk and M.F. Wheeler. Basic Microbiology, 3rd edn. J.B. Lippincott, Philadelphia, 1973.
- K. Kopecky, E. Aldova, M. Giboda, S.S. Dobahi and J. Radkovsky. J. of Hygiene, Epidemiology, Microbiology & Immunology 36 (1992) 419.
- S.M. Finegold and W.J. Martin. Bailey and Scott’s Diagnostic Microbiology, 6th edn. The C.V. Mosby Company, Missouri, 1982.
- W. K. Joklik, H.P. Willett, D.B. Amos and C.M. Wiffert (eds). Zinsser Microbiology, 19th edn. Prentice Hall, International Inc., U.S.A., 1988.
- C.M. Rademaker, L. Martinez, E. J. Perea, M. Jansze, A. C. Fluit, J.H. Glerum, et al. J. of Med. Microbiology 38 (1993)87.
- E. Maiorini, E. L. Lopez, A. L. Morrow, F. Ramirez, A. Procopio, S. Furmanski, et al. Ped. Infect. Diseases J. 12 (1993) 139.
- A.W. Allen and J.P. Wei. Southern Med. J. 85 (1992) 45.
- G. Kumarasinghe, Y.S. Lim, C. Chow and D.C Bassett. Tropical and Geographical Med. 44 (1992) 229.
- M.E. Penny, S.M. Scottland, H.R. Smith, M.M. McConnell, S.K. Knutton and R.B. Sack. Ped. Infect. Diseases J. 11 (1992) 623.
- M.S. Donnenberg, C.O. Tacket, S.P. James, G. Losonsky, J.P. Nataro, S.S. Wasserman, et al. J. Clin. Invest. 92 (1993) 1412.
- J. Bockemuh,, S. Aleksic and H. Karch. Int’l. J. of Med. Microbiology, Virology, Parasitology & Infect. Diseases 276 (1992) 189.