| Title | In vitro characterizations of antioxidant properties of Bio- Normalizer |
|---|---|
| Year | 1994 |
| Author | Yuichiro J. Suzuki and Lester Packer |
| Publisher |
April l9, 1994
In vitro characterizations of antioxidant properties of Bio- Normalizer
Yuichiro J. Suzuki and Lester Packer
Department or Molecular and Cell Biology University of California, Berkeley, California 94720
Summary—Bio-normalizer has been shown to scavenge hydroxyl radicals. In the present study, we further characterized antioxidant properties of Bio-Normalizer. Bio-Normalizer was found to contain DTNB reactive substances, possibly protein thiols, which may explain its potent scavenging activity against hydroxyl radicals. Bio-Normalizer, however, did not have any significant effects on cytochrome c reduction induced by xanthine plus xanthine oxidase suggesting that Bio-Normalizer does not scavenge-superoxide radicals or inhibits xanthine oxidase. Bio-Normalizer has no inhibitor effects on the activation of the oxidative stress- responsive transcription factor, NF-ƙB, induced by tumor necrosis factor or phorbol ester in cultured human T cells; moreover, high concentration of Bio-Normalizer (mg/ml range) exhibited a slight potentiation of tumor necrosis factor-induced NF-ƙB activation. In summary, Bio-Normalizer (1) contains DTNB reactive substances; (2) does not scavenge superoxide radicals; (3) does not inhibit xanthine oxidase and (4) does not inhibit, if not potentiates NF-ƙB activation.
Introduction
Oxidative stress has been – implicated in a variety of diseases including cancer, neurological and cardiovascular disorders and AIDS, hence the development of effective anti-oxidants have significant clinical implications. Bio-Normalizer has been shown to exhibit antioxidant action of scavenging hydroxyl radicals (Santiago et al. 1991). Thus, the antioxidant actions of Bio-Normalizer may attribute to its beneficial effects observed in various disease models in vivo.
In the present study, we further characterized antioxidant properties of Bio-Normalizer.
Materials and Methods
5 5′-dithiobis(2-nitrobenzoic acid) (DTNB) reactive substance was monitored in 60 mM KH2PO4 KOH (pH 7.4) containing 600 μM DTNB in the presence of 10 mg/ml Bio-Normalizer at 412 nm. An extinction coefficient of 13,600 M-1cm-1 was used for calculation (Suzuki et al, 1990). Superoxide radicals generated by xanthine (200 μM) plus xanthine oxidase (40 mU/ml) were measured by monitoring the reduction of ferricytochrome c (200 μM) in 60 Mm KH2PO4-KOH (pH7.4) at 550nm (Suzuki & Ford, 1991).
Jurkat T (acute human leukemia) cells were grown in RPM1- 1640 medium supplemented with 10% FCS, 1% penicillin/streptomycin and 1% glutamine at 37°C. Cells were plated in individual wells at a density of l x 106 cells/ml, and were exposed to activating agents. Bio-Normalizer was added 30 min before the addition of activators. Nuclear extraction and electrophoretic mobility shift assay were performed as described previously (Suzuki et al., 1992). Cells were harvested, washed in PBS. Pelleted cells were resuspended in buffer A, and incubated on ice for 15 min. Nonidet P-40 was added, and cells were mixed for 15 sec and centrifuged for 30 sec .The cytolosic supernatants were harvested. Pelleted nuclei were suspended in 50 μl of buffer C, mixed for 20 min, and centrifuged for 5 min. The supernatant containing nuclear proteins was harvested. Binding reaction mixtures contained -2μg-protien- of nuclear extract- 1μg poly(dI-dC), 5 – 10,000 cpm 32P-labeled probe in binding buffer [50 mM NaCl, 0.2 mM EDTA, 0.5 mM DTT, 2% glycerol and 10mM Tris-HCl (pH 7.5)]. Samples were analyzed on reactive 6% polyacrylamide gels followed by autoradiography. Double stranded oligonucleotide containing two binding sites for NF-ƙB (see sequences below) was labeled with (α-32P]dATP using Klenow Fragment.
5′ GATCCGAGGGACTTCCGCTGGGGACTTTCCAGG 3’
3′ GCTCCCCTGAAAGGCGACCCCTGAAAGGTCCCTAG 5’
Results and Discussion
DTNB assay determined that Bio-Normalizer contains reduced thiols at the level of 1.2 μmoles/g Bio-Normalizer or 32 nmoles/mg protein. These thiols, which could be the component of proteins, small molecules or both, may be responsible for the observation by Santiago et al. (1991) that Bio-Normalizer potently quenches hydroxyl radicals. They reported that 23 mg/ml Bio-Normalizer (5.1 mg/220 μl total volume) almost completely, quenched hydroxyl radicals generated by 345 μM FeSO4 (76.7 nmoles) plus 345 mM H2O2 (76.7 μmoles). Since 5.1 mg Bio-Normalizer is calculated to contain only 6.12 nmoles of SH, thiol components of Bio-Normalizer do not account for all the hydroxyl radical scavenging property.
We examined the scavenging effects of Bio-Normalizer against superoxide radical anion which is an important factor in oxygen toxicity (Suzuki & Ford, 1994). One mg/ml Bio-Normalizer (1.2 nmoles SH) did not significantly compete with 200 μM cytochrome c (200 nmoles) for superoxide radicals generated by xanthine plus xanthine oxidase generated at the rate of ~40 nmoles/min. These data suggest that thiols in Bio-Normalizer do not react with superoxide with a bimolecular kinetic rate constant of more than 1.6 x 103 M-1s-1 and other components of Bio-Normalizer as a whole do not have significant superoxide scavenging ability. It also demonstrates that Bio-Normalizer does not inhibit xanthine oxidase. Maitra and Packer (unpublished results) showed that hydrophobic fraction of Bio-Normalizer does not scavenge peroxyl radicals generated by hydrophobic radical initiator, AMVN, in hexane solution as determined by cis-parinaric acid assay, or does not inhibit lipid peroxidation of rat hepatic microsomes induced by AMVN as determined by TBARS. Thus, Bio-Normalizer does not seem to have radical scavenging ability other than to hydroxyl radicals.
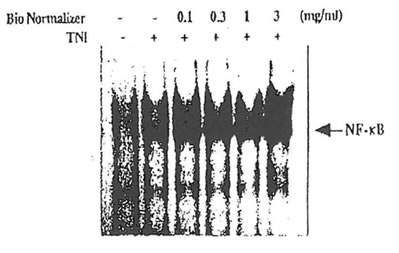
Similarly, Bio-Normalizer did not have any inhibitory action on the activation of oxidative stress-responsive transcription factor, NP-ƙB, in cultured T cells. Inhibition of NF-ƙB activation has previously been shown by various antioxidants including lipoic acid (Suzuki et al. 1992), vitamin E (Suzuki & Packer, 1993) and catechol derivatives (Suzuki & Packer, 1994). The pre incubation of Jurkat cells with Bio-Normalizer (1 μg/ml- 3 mg/ml) did not have any inhibitory actions on NF-ƙB activation induced by tumor necrosis factor (TNF; 25 ng/ml) or phorbol 12-myrestate 13-acetate (50 ng/ml) as detected by assessing the DNA binding activity of activated form of NF-ƙB. This is consistent with the report by Okamoto and Osato that Bio-Normalizer does not inhibit TNF-induced expression of CAT reporter gene fused to HIV-1 promoter which is controlled by NF-ƙB. We, moreover, observed a slight potentiation of NF-ƙB activation at high concentrations (0.1 – 3 mg/m.l) of Bio-Normalizer (Fig. 1). The lack of inhibition of NF-ƙB activation may be because active principles present in Bio-Normalizer are not penetrated in the cell.
Our observations and others demonstrate that Bio-Normalizer even at high concentrations does not exhibit significant antioxidant activity other than hydroxyl radical scavenging actions in in vitro conditions. Moreover, such high concentrations of Bio-Normalizer do not show any cytotoxicity, and even potentiate the activity of transcriptional in regulators. Bio-Normalizer has been shown to have significant effect in preventing free radical damage in vivo. These observations may be interpreted that the beneficial actions of Bio-Normalizer may only be exhibited when Bio-Normalizer metabolized in the physiological systems. That is that rather than the components of Bio-Normalizer themselves, but substances generated physiologically in response to Bio-Normalizer may be the factors responsible for the potent beneficial actions of Bio-Normalizer against oxidative stress.
References
- Santiago, L.A., J. A. Osato, M. Hiramatsu, R. Edamatsu & A. Mori. (1991) Free Rad. Biol. Med.11:379-333.
- Suzuki, Y., V. Lyall, TUL. Biber & G. D. Ford. (I990) Free Rad. Biol. Med. 9; 479-484.
- Suzuki, Y. J., & G. D. Ford. (1991) Am. J. Physiol. 261:H568- H574.
- Suzuki, Y. J., B. B. Aggarwal. & L. Packer. (1992) Biochem. Biophys. Res.Commun.189:1709- 1715.
- Suzuki, Y. J., & G. D. Ford. (1994) Free Rad. Biol. Med. 16:63-72.
- Suzuki, Y. J., & L. Packer. (1993)Biochem. Biophys. Res. Commun. 193:277- 283.
- Suzuki, Y. J., & L. Packer. (1994) Biochem. Mol. Biol Int. 32:299-305.
Figure legends
Fig. 1 Effects of Bio-Normalizer on NF-ƙB activation induced by TNF. Jurkat cells (1 x 106 cells/ml) were incubated with Bio-Normalizer for 30 min, followed by the addition of TNF (25 ng/ml) and incubation for 1 h. Nuclear extracts were isolated and electrophoretic mobility shift assays were performed as described in
Materials and Methods.