| Title | FREE RADICAL SCAVENGING ACTION OF CARICA PAPAYA LINN |
|---|---|
| Year | 1992 |
| Author | Librado A. Santiago, James Akira Osato* and Akitane Mori |
| Publisher | Neurosciences |
FREE RADICAL SCAVENGING ACTION OF CARICA PAPAYA LINN.
Librado A. Santiago, James Akira Osato* and Akitane Mori
Department of Neuroscience, Institute of Molecular and Cellular Medicine, Okayama University
Medical School, 2-5-1 Shikata-cho, Okayama 700, Japan and *Sun-0 International Inc., Gifu 500, Japan
(Received July 28, 1992; Accepted October 12, 1992)
Summary: Carica papaya Linn. (CPL) is a popular fruit and traditional medicinal herb grown in tropical countries. The possible involvement of CPL as a major component and free radical quencher in Bio-catalyzer, a commercially available natural, health food supplement, was studied by electron spin resonance/spin trapping technique. The meat, seed, pulp and the mixture of these 3 papaya parts exhibited complete sea venging actions on 1, 1-diphenyl-2-picrylhydrazyl (5.8 X 1014 spins/ml), hydroxyl (5.1 X 10 14 spins/ml) and superoxide (1.2 X 1014 spins/ml) radicals, between 25-50 mg/ml with the seed showing the strongest quenching activity. The seed at 25 mg/ml contains an equivalent 100 units/ml of superoxide dismutase-like activity required to eliminate totally the generated superoxide radicals. These findings suggest a biochemical basis for the understanding of the reported medicinal uses of CPL in various ailments.
Key words: Carla papaya Linn.-Free radical scavenger-Superoxide-Hydroxyl radicalSuperoxide dismutase-Electron spin resonance spectrometry
Naturally occurring antioxidants such as vitamin C [24], vitamin E [4, 24], isoflavones [20, 21], flavonoids [31] saponins [22], tannins [30], caffeine [27], lignans [9], alkaloids [18], and phenolic acids [3, 17, 28] have been recognized as inhibitors of free radicals and lipid peroxidation.
Carica papaya Linn. (CPL) is a small, fast-growing tree and widely eaten fruit grown in tropical countries. Philippine ethnomedical information on this popular medicinal plant reveals that the fruits, stems, leaves and roots may be used as diuretics, emmenagogue, laxative, anthelmintics, antiasthmatic, antidyseptic, antirheumatic, rubefacient, stomachic, tonic, poultice, and abortifacient, among others [23]. The fruit in particular is used as laxative and cosmetic, cure for verrnifudge and enlargement of liver and spleen, freckles and cancerous growth [23].
To reassess the purported therapeutic action of CPL and its active participation as a major component of Bio-catalyzer (BC), a commercially available natural, health food supplement with proven hydroxyl radical scavenging property [25, 26], we examined its free radical quenching activity by electron spin resonance (ESR)/spin trapping technique, namely the meat, seed, pulp and the mixture of these 3 papaya parts against 1,1- diphenyl-2-picrylhydrazyl (DPPH), hydroxyl and superoxide radicals. We also analyzed the superoxide dismutase (SOD)-like activity in crude preparations of papaya.
METHODS
Chemicals: DPPH, hypoxanthine, and diethylene-triaminepentaacetic acid (DETAPAC) and SOD (bovine serum; 2,800 units/mg were obtained from Sigma Chemical Co. (St Louis, MO, USA); xanthine oxidase was from Boehringer-Mannheim (Germany) and 5,5′-dimethyl-1-pyrroline-Noxide (DMPO) was from Daiichi Pure Chemicals Co., Ltd. (Tokyo, Japan). All other chemicals and reagents used were of the highest grade available from commercial suppliers.
Plant Sample: A 2-3 week-old Philippine variety of CPL measuring 14 x 7 cm was used. The fresh weight basis of the meat, seeds and pulp papaya were osteurized with water.
Analysis of free radicals: The details of the analysis of DPPH, hydroxyl, and superoxide radicals by ESR spectrometry (JEOL, JES-FEIXG, Tokyo, Japan) were given in previous reports [24-26].
Analysis of SOD: Analysis of SOD activity was performed by ESR spectrometry [15, 16]. Osteurized papaya juice was centrifuged at 2,000 rpm for 15 min and the supernatant was used. Fifty microliters of 2 mM hypoxanthine, 35 µl of 11 mM DETAPAC, 50 µl of the supernatant, 15 µl of DMPO, and 50 µl of freshly prepared xanthine oxidase (81.6 µl in 5 rnl of phosphate buffer, pH 7.8) were mixed in a 169 µl-volurne capacity special flat cell (JEOL, Tokyo, Japan) and the superoxide spin adduct (DMPO-OOH) was recorded exactly after 1 min of mixing. A standard SOD curve was prepared from different diluted concentrations of SOD in 0.1 M sodium phosphate buffer (pH 7.4). Manganese oxide was used as an internal standard. The spin number was calculated from the ratio signal height intensity of 2,2,6,6-tetramethyl-4-hydroxyl piperidine-1-oxyl with known spin number. The SOD activity was expressed as unit/ml.
Statistical Analysis: Statistical analysis was performed using Student’s t-test.
RESULTS
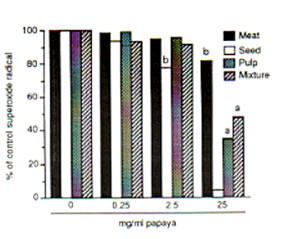
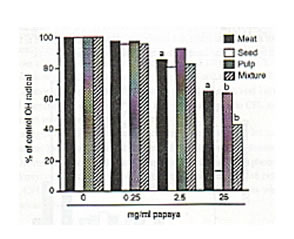
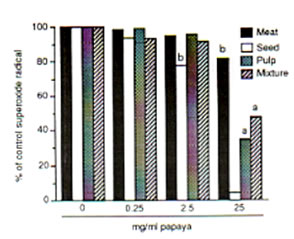
The meat, seed, pulp and the mixture/combination of these three papaya parts exhibited scavenging actions on DPPH, hydroxyl and superoxide radicals at varied potencies with the papaya seed showing strongest radical-inhibiting property. Against DPPH radical, the scavenging action of the papaya parts were first observed at 0.5 mg/ml each as about 5% to 11% of DPPH radicals (5.84 X 1014 spins/ml) was eliminated. Fifty mg/ml each of papaya seed and pulp resulted to a 99% and 97% scavenged DPPH radical, respectively (Fig. 1). Papaya seed and mixture·inhibited the hydroxyl radicals (5.1 x 10’4 spins/ml) generated from the Fenton reaction by 85% and 57%, respectively at 25 mg/ml each, while only 47% was quenched by the pulp and meat (Fig. 2). The superoxide radicals (1.22 x 1QI4 spins/ml) were 95%, 66% and 52% controlled by the papaya seed, pulp and mixture, respectively at 25 mg/ml each (Fig. 3). At 25 mg/ml, the seed gave the highest SOD-like activity equivalent to 100 units/ml of the enzyme required to dismutate the superoxide radicals in hypoxanthine-xanthine oxidase generating system (Fig. 4).
Fig. 1. Dose-dependent scavenging action of different parts of unriped papaya fruit on 1,1- diphenyl-2-picrylhydrazyl (5.84 X 1014 spins/ml). ESR settings were: magnetic field, 334 ± 10 mT; response, 0.3 sec; sweep time, 0.5 min; amplitude, 3.2 X 1000, modulation amplitude, 0.08 mT; and power, 8 mV. Values are means of 4-6 determinations. aP < 0.05, bp < 0.025 versus control.
Fig. 2. Dose-dependent scavenging action of different parts of unriped papaya fruit on hydroxyl radicals (4.63 X 1015 spins/ml) generated from Fenton reaction. ESR settings were: magnetic field, 334 ± 5 mT; response, 0.1 sec; sweep time, I min; amplitude, 4 X 100, modulation amplitude, 0.08 mT; and power, 8 mV. Values are means of 4-6 determinations. aP < 0.05, bp < 0.025 versus control.
Fig. 3. Dose-dependent scavenging action of different parts of unriped papaya fruit on superoxide radicals (3.78 X 1014 spins/rnl) generated from hypoxanthine-xanthine oxidase system. ESR settings were: magnetic field, 335 ± 5 mT; response, 0.1 sec; sweep time, 2 min; amplitude, I X 1000, modulation amplitude, 0.08 mT; and power, 8 mV. Values are means of 4-6 determinations. •P < 0.05, bp < 0.025 versus control.
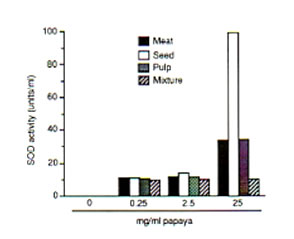 Fig. 4. Superoxide dismutase (SOD)-like activity in different parts of unriped papaya fruit that scavenges superoxide radicals generated from hypoxanthine-xanthine oxidase system. ESR settings were the same as in Fig. 3. Values are means of 4-6 determinations.
Fig. 4. Superoxide dismutase (SOD)-like activity in different parts of unriped papaya fruit that scavenges superoxide radicals generated from hypoxanthine-xanthine oxidase system. ESR settings were the same as in Fig. 3. Values are means of 4-6 determinations.
DISCUSSION
Antioxidant properties of compounds present in “natural medicines” of plant origin have been proposed as useful clinical agents in the prevention or attenuation of diseases; plants with anticancer and antimalarial [5], antigenotoxic [18], anti-inflammatory [6-8], and antiviral and anti AIDS (HIV) [12-14] have found applications in the “third medicine”.
Suggestions have been made that a major component CPL of BC; a health food supplement with proven free radical scavenging action and which inhibited a) neuronal peroxidative damage in rat epileptic focus [25], b) thiobarbituric acid-reactive substances in aged rat brain while increasing antioxidant enzyme expression of mitochondrial and cytosolic SOD (unpublished data), and c) hydroxyl radical-mediated synthesis of methylguanidine in aged rat brain (unpublished report); may be responsible for these significant observations. By ESR/spin trapping technique, the scavenging action of meat, seed, pulp, and mixture of these 3 papaya parts against DPPH, hydroxyl and superoxide radicals was unequivocably demonstrated – pointing to an involvement of CPL in the inhibition of, or preferential reaction with the radical species. Among the papaya parts studied, it appears that the seed has the highest capability in eliminating the 3 radicals. The SOD-like activity detected in the 3 papaya parts mitigate the oxidative stress exerted by the superoxide radicals. It may be noted that Fe-SOD has been purified from tomato and mustard; Mn-SOD from maize and pea; and Cu-, Zn-SOD from green pea, maize, wheat germ, spinach chloroplast, pea and corn seedlings, cucumber and green pepper [11]. This is an important observation since toxicity by reactive oxygen species, in particular hydroxyl and superoxide radicals, has been implicated as likely causes of cancer, coronary heart disease, and aging, among others [ 1, 10, 11, 29]. The in vitro inhibiting effects of CPL against free radicals, in particular its fruits, may be attributed to saccharose (0.85%), glucose (2.6%), and levulose (2.1%) [23] which contain adjacent -OH groups; carboxylic acids such as citric acid, levulinic acid and malic acid [23]; and vitamins A, B, and C [23]. Among these compounds, vitamin C [24], glucose and citric acid [11] are proven free radical quenchers. Carotenoids, which may also be present in CPL as vitamin A precursors, are involved in antioxidant defenses in vivo [2]. Our fmdings suggest that CPL as an antioxidant may protect the redox integrity of foods and organisms by preventing food spoilage and the onset of free radical-related diseases. This may be possible thru its SOD-mimic activity to dismutate superoxide radicals and its phenolic groups’ ability to eliminate hydroxyl radical, chelate metals and/or break chain peroxidative events. As a major component of BC, CPL represents an important clinical agent supporting its widely recognized pharmacological uses in various ailments.
REFERENCE
- Ames, B.N. (1989) Endogenous DNA damage as related to cancer and aging. Mutation Res. 214:41-46.
- Burton, G.W. (1989) Antioxidant action of carotenoids, J.Nutr. 119:109-111.
- Cholbi, M.R., Paya, M. and Alcaraz, M.J. (1991) Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation. Experientia 47: 195-199.
- Chow, C.K. and Drapper, H.H. (1974) Oxidative stability and antioxidant activity of the tocopherols in corn and soybean oils. Int. J. Vitam. Nutr. Res. 44:396-403.
- Cordell, G.A., Pezzuto, J.M., Lin, L.Z., Lin, L.J., Shie, H.L., Mar, W., Likhitwitayawuid, K., Angerhofer, C.K., Lankin, D.C., Xue, L. and Johnson, M.E. (1992) Anticancer and antimalarial agents from plants. In: Abstract of Seventh Asian Symposium on Medical Plants, Spices, and other Natural Products, Manila, Philippines.
- Croft, K.D., Beilin, L.J. and Ford, G.L. (1987) Differential inhibition of thromboxane B2 and leukotriene B4 biosynthesis by two naturally occuring acetylenic fatty acids. Biochim. Biophys. Acta 921: 621-624.
- Croft, K.D., Beilin, L.J., Vandongen, R. and Mathews, E. (1984) Dietary modification of fatty acid and prostaglandin synthesis in the rat. Effects of variations in the level of dieatary fat. Biochim. Biophys. Acta 795: 196-207.
- Croft, K.D., Codde, J.P., Barden, A., Vandongen, R. and Beilin, L.J. (1985) Onset of changes in phospholipid fatty acid composition and prostaglandin synthesis following dietary manipulation with n-6 and n-3 fatty acids in the rats. Biochim. Biophys. Acta 834:316-323.
- Faure, M., Lissi, E., Torres, R. and Videla, L.A. (1990) Antioxidant activities of lignans and flavonoids. Phytochemistry 29: 3773-3775.
- Fridovich, I. (1978) The biology of oxygen radicals. Science 201: 875-880.
- Halliwell, B. and Gutteridge, J.M.C. (1989) Free Radicals in Biology and Medicine, Clarendon
Press, Oxford. - Hudson, J.B., Lopez-Bazzochi, I. and Towers, G.H.N. (1991) Antiviral activities of hypericin. Antiviral Res. 15:101-112.
- Hudson, J.B. and Towers, G.H.N. (1991) Therapeutic potential of plant photosensitizers. Pharmac. Ther. 49:181-222.
- Hudson, J.B. and Towers, G.H.N. (1992) Anri-AIDS virus (HIV) compounds from plants. In: Abstract of Seventh Asian Symposium on Medicinal Plants, Spices, and other Natural Products, Manila, Philippines.
- Hiramatsu, M. and Kohno, M. (1987) Determination of superoxide dismutase activity by electron spin resonance spectrometry using the spin trap method. JEOL News 23A: 7-9.
- Hiramatsu, M., Kohno, M., Mitsuta, K. and Mori, A. (1992) Increased superoxide dismutase activity in aged human cerebrospinal fluid and rat brain determined by electron spin resonance spectrometry using the spin trap method. J. Neurochem. 58:1160-1164.
- Larson, R.A. (1988) The antioxidants of higher plants. Phytochemistry 27: 969-978.
- Lim-Sylianco, C.Y., Mallorca, S.R., Balboa, J. and Sylianco-Wu, L. (1992) Antigenotoxic activity of coconut oil in bone marrow cells of mice given azaserine, benzo (a) ptyrene, dimethylnitrosamine, dimethylhydrazine, methylmethanesulfonate and tetracycline. In abstract of Seventh Asian Symposium on Medicinal Plants, Spices, and other Natral Products, Manila, Philippines.
- Martinez, L.A., Rios J.L., Paya, M. and Alcaraz, M.J. (1992) Inhibition of nonenzymic lipid peroxidation by benzylisoquinoline alkaloids. Free Radical Biol. Med. 12: 287-292.
- Naim, M., Gestetner, B., Bondi, A. and Birk, Y (1976) Antioxidative and anti-hemolytic activities of soybean isoflavones. J.Agric. Food Chem. 24: 1174-1177.
- Naim, M., Gestetner, B. Kirson, I., Birk, Y. and Bondi, A. (1973) A new isoflavone from soya beans. Phytochemistry 12: 169-170.
- Ohminami, H., Kimura, Y., Okuda, S. and Arichi, S. (1984) Effects of soya saponins on liver injury induced by highly peroxidized fats in rats. Planta Med. 50: 440-441.
- Quisumbing, E. (1978) Medicinal Plants of the Philippines, Katha Publishing, Manila.
- Santiago, L.A., Hiramatsu, M., and Mori, A. (1992) Japanese soybean paste miso scavenges free radicals and inhibits lipid peroxidation. J.Nutr. Sci. Vitaminol. 38: 297-304.
- Santiago, L.A., Osato, J.A., Hiramatsu, M., Edamatsu, R. and Mori, A. (1991) Free radical scavenging action of Bio-catalyzer α.ρNo.11 (Bio-normalizer) and its by-product. Free Radical Biol. Med. 11: 379-383.
- Santiago, L.A., Osato, J.A., and Mori, A. (1992) Stability of the hydroxyl radical scavenging components of the health food “Bio-normalizer”. Med. Sci. Res. 20: 27-28.
- Shi, X. and Dalal, N.S. (1991) Antioxidant behavior of caffeine: Efficient scavenging of hydroxyl radicals. Fd. Chem. Toxic. 29:1-6.
- Toda, S., Kimura, M. and Ohnishi, M. (1991) Effects of phenolcarboxylic acids on superoxide anion and lipid peroxidation induced by superoxide anion. Planta Med. 57: 8-10.
- Totter, J.R. (1980) Spontaneous cancer and its piossible relationship to oxygen metabolism. Proc. Natl. Acad. Sci. USA 77: 1763-1767.
- Uchida, S., Edamatsu, R., Hiramatsu, M., Mori, A., Nonaka, G., Nishioka, I., Niwa, M. and Ozaki, M. (1987) Condensed tannins scavenge active oxygen free radicals. Med. Sci., Res. 15:831-832.
- Yuting, C., Rongliang, Z., Zhongjian, J. and Yong. J. (1990) Flavonoids as superoxide scavengers and antioxidants. Free Radical Biol. Med. 9: 19-21.