| Title | PROTECTIVE EFFECT OF BIO- NORMALIZER AGAINST ASBESTOS- INDUCED LUNG INJURY. |
|---|---|
| Year | |
| Author | Ludmila G.Korkina, Natalia E .Abramova, Elena A Ostrachovich, Elena V. Mikhalchick, Veronica A. Lipatova, and I.B.Afanas’ev |
| Publisher |
1 . PROTECTIVE EFFECT OF BIO- NORMALIZER AGAINST ASBESTOS- INDUCED LUNG INJURY.
Ludmila G.Korkina1, Natalia E .Abramova1, Elena A Ostrachovich2, Elena V. Mikhalchick1, Veronica A. Lipatova1, and I.B.Afanas’ev2
1 – Russian Medical University, Lab. of Occupational Lung Diesease, Ostrovityanova 1, Moscow 117437, Russia.
2 – Vitamin Research Institute, Nauchny pr. 14A, Moscow 117860, Russia
Introduction
It has been shown that exposure to asbestos fibers leads to increasing risk of various malignancies (bronchogenic carcinoma, mesothelioma, and gastrointestinal tumors), the development of interstitial fibrosis (asbestosis) and unspecific inflammation in the lung tissue (alvcolitis) [1,2]. We [3] and others [4] have previously shown that asbestos fibers stimulate oxygen radical production by phagocytes (neutrophils and macrophages). It is likely that the release of reactive oxygen species (ROS) by phagocytes is the predominant way by which ROS are involved in an inflammatory process and fibrogenesis [5,6]. On these grounds we suggested that the study of the effects of BN administration to experimental animals with lung injury induced by asbestos fibers may be an important test of BN anti-inflammatory and antifibrotic activities.
Materials and Methods
Animals.
Experiments were carried out on male Wistar rats of body weight ranging from 150 to
200 g. The animals were kept on standard chow diet and water αd libitum under normal laboratory conditions.
Animal model of lung injury.
The rats were divided into 4 groups, each consisting of 24 animals. Rats of group I were given 1 ml of physiological saline intratracheally (control group). Animals of group II were injected intratracheally with 15 mg of chrysotile asbestos fibers and 10 mg of Bio ormalizer suspended in 1 ml of physiological saline. Animals of group III were fed daily with 50 mg of Bio-normalizer and standard diet during 7 days, after that they were given 15 mg of chrysotile asbestos fibers intratracheally, and the animals were fed again with 50 mg of Bio-normalizer for next 7 days. Group IV: animals were injected with 15 rng of asbestos fibers. The animals were sacrificed at 3d, 7th, 14th, and 28th days after the intratracheal injection of NaCl, asbestos fibers, or asbestos fibers with Bio-normalizer.
Isolation of alveolar cells
Alveolar macrophages and neutrophils were prepared by pulmonary lavage using the slightly modificated method [7]. Briefly, animals were sacrificed by intraperitoneal injection of sodium thiopental, 50 mg/kg. The trachea was cannulated and the lungs were lavaged five times with 10 ml of HBSS, pH 7.4, at 37°C. The lavage fluid was filtered through a nylon cloth. The filtrate was centrifuged at 300xg for 10 min. Cell content was determined in a hemocytometer and differential count was performed microscopically in the cell smears. To separate mononuclear and polymorphonuclear cells, cell pellet was resuspended in 1 ml of HBSS, layered carefully on Ficoll-Paque gradient (ρ = 1.077 kg/l), and centrifuged at 600xg for 40 min. The upper layer consisted of mononuclear cells (macrophages and lymphocytes) and the pellet contained granulocytes (mainly neutrophils). Macrophages were identified by staining for nonspecifice sterase. Macrophage and neutrophil preparations were >95% pure and 98% of the cells excluded trypan blue. The isolated cells were resuspended and stored at 4°C in HBSS supplied with 5% fetal calf serum.
The lung preparation
After broncho-alveolar lavage preparation, the lungs were removed, weighted, and part of them was used for hydroxyproline and lipid peroxidation determination. The remaining tissue was dried and weighted again for the wet/dry weight ratio determination.
Lipid peroxidation of rat lung homogenate
The lung tissue was dissected into small pieces, poured through 3mm holes for the fibrotic wall separation, and homogenized thoroughly. 10 mg of homogenate sample were incubated with 50 μΜ FeCI3 and 800 μΜ ADP in 1 ml of 0.1 M phosphate buffer (pH7.4) at 37°C. The reaction was started by adding 40 μl of 7.5 mM NADPH in phosphate buffer. After 30 min incubation, the reaction was stopped by adding 1 ml of 15% trichloroacetic acid and 0.1 m1 of 10 μM naphtol solution in ethanol. Then, 1 ml of 0.375% TBA solution was added, and reaction mixture was boiled for 15 min. After sedimentation of precipitated proteins by centrifugation, the content of TBA reactive products was determined by measuring the absorbance at 535 nm.
Chemiluminescence assay
CL measurements in a Luminometer mod. 1251 (LKB, Sweden) were monitored at 37° and continuous mixing on a programmed IBM computer. All experiments were carried out in duplicates. Each point was a mean of 3 or 4 independent measurements.
A sample of 5×105 cells, 400 μM luminol or lucigenin, and 0.5 ml of HBSS pH 7.4 were placed 1-ml polysterene vials. The vial was then placed in the thermostated detection chamber of a luminometer. After measurement of background for 5 min, CL was activated by introducing of PMA (10 ng) or opsonized zymosan (100 ml). The CL – response was measured as a mean mV signal over 10-s intervals for a 10-min period. Amplitude of the CL-response was defined as a difference between maximal intensity of activated CL and intensity of spontaneous CL and expressed as m V/ 106 cells.
The determination of hydroxyproline in rat lung tissue
The hydroxyproline content was measured by slightly modified method of stegemann [8]. In short, a sample of dried lung tissue (20 mg) was hydrolized with 6 M hydrochloric acid (1 ml) at 100°C for 24 hours. After filtration, neutralization, and vaporization of water, dry residue was dissolved in 1 ml of distilled water. A sample was incubated with 1 ml of 0.03 M chloroamine B solution prepared in the mixture of acetate-citrate buffer + propyl alcohol for 5 min at room temperature. Finally, the reaction mixture was treated with 1 m1 of 5% p-dmethylaminebenzaldehyde at 60°C for 20 min and the content of hydroxyproline was determined spectrophotometrically at 558 nm.
Statistical analysis.
All data are present as mean ± SEM Data were analyzed by analysis of variance followed by paired or unpaired t-test for multiple group comparison. A P value less than 0.05 was significant.
Results and Discussion
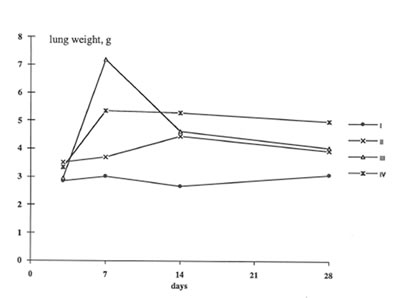
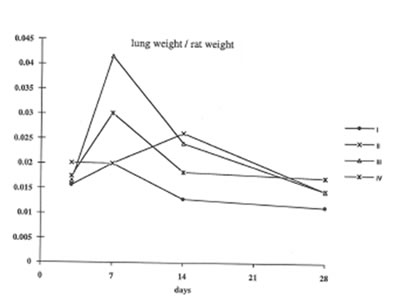
In present work we have studied the in vivo effects of BN on the lung inflammation and fibrosis induced by chrysotile asbestos exposure. We compared some objective symptomes of lung injury in the rats with and without BN administration; in addition, the specific parameters characterized the rat organism’s free radical status, namely, lipid peroxidation in the lung tissue and oxygen radical production by the pulmonary cells were determined. It was found that BN administration diminished the pathologic changes in the rat lungs induced by asbestos fibers instillation. Thus, wet lung weight and the lung weight/rat weight ratio decreased in the asbestos-injected rats under BN administration (Group 2 and 3) in comparison with those for Group 4 and approached the parameters of control animals (Group 1) on 14th and 28th days ( Figs 1 and 2).
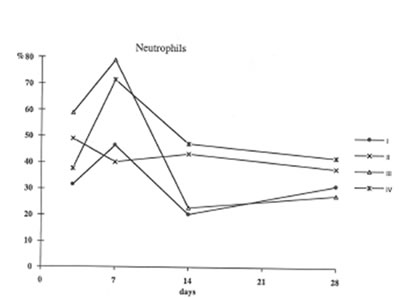
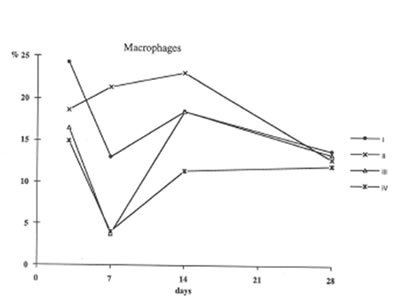
Furthermore administration to asbestos injected rats decreased recovery of Neutrophils into broncho-alveolar space (Fig. 3) and enhanced that of macrophages (Fig4). These processes (the suppression of neutrophil attachment to immflamatory loci and the enhancement of macrophage recruitment and their phagocytic capacity) apparently determine the beneficial effects of BN on aseptic inflammation. It seems of utmost importance that similar effect of BN has been already shown by us in the in the inflammation model induced by intraperitoneal dextran injection [9]. Therefore one may conclude that the suppression of neutrophil-defined stage and the stimulation of macrophage stage of inflammation can explain an universal normalizing effect of BN in a variety of inflammatory pathologies.
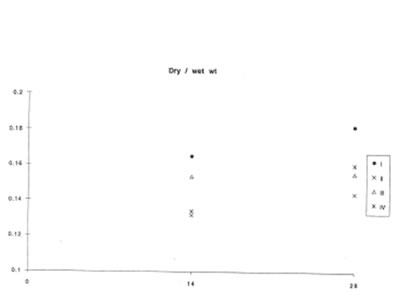
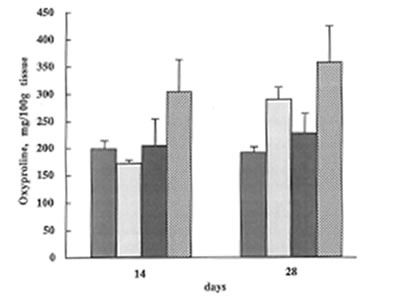
At the same time, BN did not affect lung vascular permeability which was usually enhanced during inflammatory process. As is seen from Fig.5 BN administration did not change significantly lung edema defined by the wet/dry lung weight ratio. It is now well established that lung inflammation often precedes the development of lung fibrosis [5,6]. The amount of hydroxyproline in the lung tissue corresponds to the intensity of asbestos-associated fibrosis. We found that BN administration drastically decreased hydroxyproline formation on 28th day after asbestos exposure [Fig. 6].
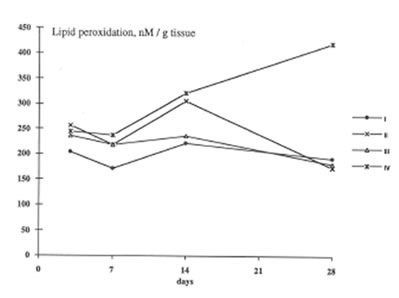
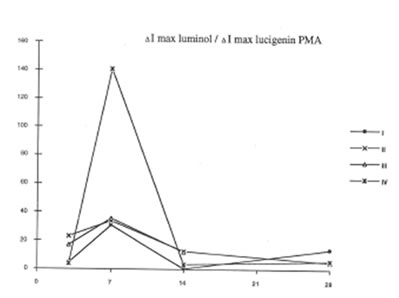
BN exhibited profound inhibitory action on free radical formation in lung tissue. We studied two free radical processes in the lung: lipid peroxidation in the lung tissue and the release of oxygen radicals by pulmonary phagocytozing cells. As is seen from Fig.7, BN administration to asbestos-injected rats completely annihilated the asbestos-induced enhancement of lipid peroxidation in the lung homogenate on 28th day after the beginning of experiment. At the same time, the BN effect on oxygen radical production by lung phagocytes was much more complicated. On a whole, as it should be expected, BN administration decreased spontaneous and PMA- or zymosan-stimulated luminol- and lucigenin-amplified CL produced by the cells of bronchoalveolar lavage from asbestos-injected rats, although this inhibitory effect was not so prominent as in the case of dextran associated inflammation [9]. On the other hand, BN was found to be a very effective inhibitor of hydroxyl radical formation (determined by luminol-amplified CL) without affecting substantionally superoxide production (measured by lucigenin-amplified CL) Owing to that, the luminol-amplified CL lucigenin-amplified CL ratio, which achieved a very high level on 7th day in asbestos-treated rats, dropped to a normal value in the rats after BN administration. (Fig. 10). We have shown earlier [10,11] that the overproduction of hydroxyl radicals is typical for various free radical-mediated disorders. Therefore, BN administration to the rats with asbestos- induced lung injury permits to suppress the formation of the most toxic free radicals, which are certainly an important initiation factor of the lung damage.
Conclusions
- Intratracheal and per os BN administration to the rats with asbestos- induced lung damage ameliorated substantially the inflammation and fibrosis in lung tissue.
- BN administration suppressed two important free radical-mediated destructive processes in injured lung: lipid peroxidation and oxygen radical release from pulmonary phagocytes.
- BN was able to suppress the overproduction of highly reactive hydroxyl radicals by lung phagocytes.
- The above findings indicate that BN is able to exhibit beneficial effects in the prevention and treatment of asbestos-associated lung injury.
References
- Becklake, M.R., Asbestos-related diseases of the lung and other organs: Their epidemiology and implications for clinical practice, Am.Rev.Respir.Dis. 114,187-227(1976).
- Craighead, J.E. and Mossman, B.T., The phatogenesis of asbestos-associated diseases,N.Engl.J.Med. 306, 1446-1455 (1982).
- Velichkovskii, B.T.,Cheremisna Z.P., Suslova, T.B., Korljna, L.G., Olenev, V.I., Molecular mechanism of asbestos biological activity, Gigiena Truda (russ) N9, 5-10 (1986).
- Takeuchi, T., Morimoto. K., Kosaka, H., and Shiga, T., Spin trapping of superoxide released by opsonized asbestos from human promyclocytic leukemia cell line, HL60, Biochem.Biophys.Res.Commun. 194, 57-64 (1993).
- Haslam, P.L.,Dewar, A., Butchers, P., Primett, Z.S., Newman-Taylor, A., and Turner-Warwick, M., Mast cells, atypical lymphoctes, and neutrophils in bronchoalveolar lavage in extrinsic allergic alveolitis Comparison with other interstitial lung diseases, Am.Rev.Respir.Dis. 135, 35-47 (1987).
- Holgate, S., Contribution of inflammatory mediators to the immediate asthmatic reaction, Am. Rev.Res. Dis. 135, S57-S62 (1987).
- Forman, H.J., Dorio, R.J., and Skelton, D.C., Hydroperoxide-induced damage to alveolar macrophage function and membrane integrity: alterations in intracellular-free Ca2+ and membrane potential Arch. Biochem. Biophys. 259, 457-465 (1987).
- Stegeman, H.,Mikrobestimmung von hydroxyprolin mit chloramin T und p-dimetylaminobensaldehyd, Hoppi-Seylers Z Physiol. Chem. 311, 41-45 (1958).
- Osato, J.A., Afanas’ev, I.B., Cherernisina, Z.P., Suslova, T.B., Abramova, N.E., Mikhalchik E.V, Deeva, I.B., Santiago, L.A., and Korkina, L.G., Bio-normalizer as a modulator of phagocytosis and free radical production by murine inflamed Neutrophils and macrophages, in press.
- Korkina, L.G.,Samochatova, .E.V., Maschan, A.A, Suslova. T.B.,Cheremisina, Z.P., and Afanas’ev, I.B., Released of active oxygen radicals by leukocytes of Fanconi anemia patients J.Leukocyte Biol.52,357-262 (1992).
- Korkina, L.G., Afanas’ev, I.B. and Diplock, A.T.,Antioxidant therapy in children affected by irradiation from the Chernobyl nuclear accident, Biochem.Soc.Trans. 21,
314S (1993).
Legends to Figures
Figure 1
The change in the rat lung weight during the experiments.
Group 1- Control; Group 2- Asbestos + BN; Group 3- preliminary feeding with BN+ asbestos Group 4- Asbestos.
Figure 2
The change in the lung weight /rat weight ratio during the experiment (the same symbols as in Figure 1).
Figure 3
The recovery of neutrophils into broncho –Alveolar space (the same symbols as in Figure 1.).
Figure 4
The recovery of macrophages into broncho – Alveolar space (the same symbols as in Figure 1).
Figure 5
The change in the lung dry weight/wet weight ratio during experiment (the same symbols as in Figure 1).
Figure 6
The change in the hydroxyproline content during the experiment ( the same symbols as in Fig.1).
Figure 7
Lipid peroxidation in the lung tissue (the same symbols as in Figure 1).
Figure 8
The luminol-amplified CL/Lucigenin –amplified CL ratio for PMA- stimulated cells or broncho-alveolar lavage.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8