| Title | ANTIOXIDANT ACTIVITY OF JAPANESE SKIN CARE PRODUCT RUNN-RUNN |
|---|---|
| Year | 1995 |
| Author | Librado A. Santiagoa,b, James Akira Osatob,c and Akitane Moria |
| Publisher | Magnetic Resonance in Medicine |
Manuscript on
Antioxidant Activity of Japanese Skin Care Product Runn-Runn
Librado A. Santiagoa,b, James Akira Osatob,c and Akitane Moria
aDepartment of Neuroscience, Okayama University Medical School, Okayama 700, Japan, bOsato Research institute, Gifu 500, Japan and cThe United Graduate School of Agricultural Science, Gifu University, Gifu 500, Japan
Submitted for the
Proceedings on International conference on bio-Radicals Detected by ESR Spectroscopy
June 12-16, 1994
Yamagata, Japan
Antioxidant Activity of Japanese Skin Care Product Runn-Runn
Librado A. Santiagoa,b, James Akira Osatob,c and Akitane Moria
aDepartment of Neuroscience, Okayama University Medical School, Okayama 700, Japan, bOsato Research institute, Gifu 500, Japan and cThe United Graduate School of Agricultural Science, Gifu University, Gifu 500, Japan
To demonstrate the beneficial effects of Runn-runn, a brown granular face wash and body skin care product fermented from rice bran, Bio-normalizer and other plant extracts, we studied its free radical scavenging action using electron spin resonance (ESR) spectrometry/spin trapping technique. We reported that rice bran may be applicable in cosmetic industry because of its antioxidants such as a-tocopherol, oryzanol, isovitexin, squalene and other phenolic compounds (1-6) some of which are known components in cosmetics. Ultraviolet irradiation has been widely implicated to damage the skin and increase the hydroperoxide content and the collagen synthesis of the skin.
Materials and Methods
1,1-diphenyl-2-picrylhydrazyl (DPPH), hypoxanthine, diethylenetriamine pentaacetic acid and SOD were obtained from Sigma Chemical Co., St. Louis, MO; xanthine oxidase was from Boehringer GmbH, Mannheim; 5,5’-dimethyl-1-pyrroline-N-oxide (DMPO) was from Daiichi Pure Chemicals Co. Ltd., Tokyo; vitamin C from Wako Pure Chemical Ind. Ltd., Osaka. All other reagents used were of the highest grade available from commercial suppliers. Male adult Sprague-Dawley rats from Clea Japan Inc., Tokyo weighing 250-300 g were housed at 25ºC and humidity of 50 ± 5% and were fed ad libitum with a standard diet for 7 days prior to sacrifice by cervical decapitation. The cerebral cortex was dissected and homogenized (0.05% w/v) in 0.1 M Tris-HCl buffer, pH 7.4. Runn-runn, a product of Sun-O International Inc., Gifu, Japan was homogenized and centrifuged at 1,000 rpm for 5 min at 4ºC. By ESR spectrometry/spin trapping (JEOL, JES-FE1XG, Tokyo), the reactions of the supernatant with the different generated free radicals such as DPPH in ethanolic solution, hydroxyl radical from Fenton reagent, superoxide radical from hypoxanthine-xanthine oxidase, and carbon-centered radical from rat brain homogenate incubated at 37ºC for 15 min with 2 mM each of ferrous ions and ascorbic acid were tested as stated in previous reports (6-7).
Results and Discussion
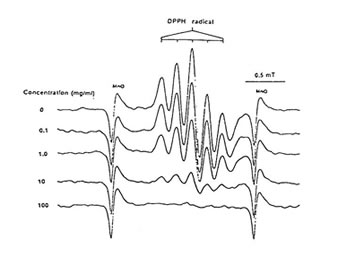
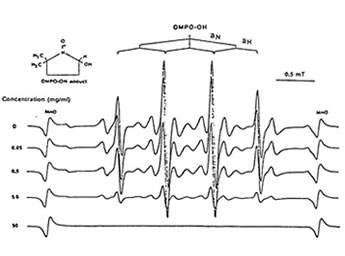
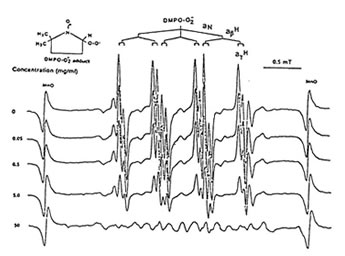
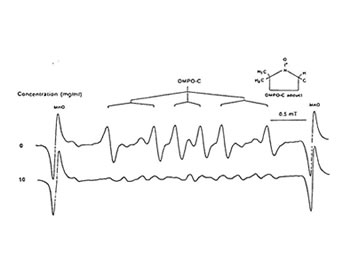
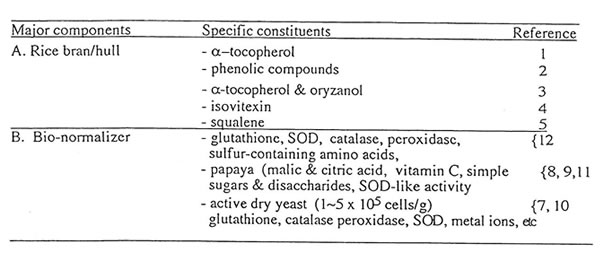
Figure1 shows that 100 mg/ml of Runn-runn scavenged 99.0% of DPPH (0.28 x 1014 spins/ml) in ethanolic solution while 50 mg/ml quenched 99.2% of DMPO-OH spin adducts (3.14 x 10 spins/ml) generated from Fenton reaction (Fig. 2), and 97.03% of DMPO-O2–spin adducts (1.12 x 1014 spins/ml) produced by the hypoxanthine-xanthine oxidase system (Fig. 3). 10 mg/ml Runn-runn inhibited 85.0% of carbon-centered radicals (DMPO-C spin adducts) (6.6 x 1012 spins/ml) generated in rat brain homogenate (Fig. 4). The free radical scavenging properties of Runn-runn may be ascribed to its two major components such as rice bran and Bio-normalizer. Table 1 shows the specific antioxidants in rice bran such as a-tocopherol, oryzanol, phenolic compounds, isovitexin, squalene, superoxide dismutase (SOD)-like activity, and Bio-normalizer which contains malic and citric acids, vitamin C, simple sugars, SOD, sulfhydryl amino acids, glutathione, peroxidase, catalase, and yeasts, among others. The data suggest that Runn-runn, used in skin care treatment may play a role as an antioxidant against potentially harmful effects of ultraviolet irradiation and other photosynthetic systems that can cause accelerated aging and excessive oxidations and collagen synthesis of the skin.
Fig. 1 Dose-Dependent scavenging action of aqueous phase of Runn-runn on ESR spectra of 1,1-diphenyl-2-picrylhydrazyl (0.28 x 1014 spins/ml). ESR setting: band, X-band; power, 0.08 mT; modulation frequency, 100 kHz; and scan rate, 0.5 min.
Fig. 2. Dose-dependent scavenging action of aqueoues phase of Runn-runn on DMPO-OH spin adducts (3.14 x 1014 spins/ml) generated from Fenton reaction. ESR setting: band, X-band; power, 0.08 mT; modulation frequency, 100 kHz; and scan rate, 1 min. The hyperfine splitting constants are aN = 15.1G = abH
Fig. 3. Dose –dependent scavenging action of aqueous phase of Runn-runn on DMPO-O2 spin-adducts (1.12 x 1014 spins/ml) generated from hypoxanthine-xanthine oxidase system. ESR setting: band, X-band; power,0.08 mT; modulation frequency, 100 kHz; and scan rate, 2min. The hyperfine splitting constants are aN = 14.6 G and agH = 1.2 G.
Fig. 4. Effect of aqueous phase of Runn-runn on DMPO-C spin adducts (6.6 x 1012 spins/ml) generated from incubated rat brain homogenate induced with 2mM each of FeCl2 and ascorbic acid. ESR setting: band, X-band; power, 0.08 mT;modulation frequency, 100 kHz;and scan rate, 0.5 min. The hyperfine splitting constants are aN=15.5 G and aH=23.0G
Table 1. Possible antioxidants in Runn-runn
References
- Changbumrung, S., Buavatana, T. and Migasena, P. 1980. Int. J. Vitam. Nutr. Res., 50:242-246
- Tajima, K., Sakamoto, M., Okada, K. et al. 1983. Biochem. Biophys. Res. Comm., 115:1002-1008.
- Ramarathnam, N., Osawa, T., Namiki, M. et al. 1986. J. Sci. Food Agric. 37:719-726.
- Ramarathnam, N., Osawa, T., Namiki, M. et al. 1989. J. Agric. Food Chem., 37: 316-319
- Windholz, M. and Budavari, S. (eds.) 1976. The Merck Index, Merck & Co. Inc., USA, pp. 8547-8548.
- Santiago, L.A. and Mori, A. 1992. Med. Sci. Res., 20:827-828.
- Santiago, L.A., Osato, J.A., Hiramatsu, M., Edamatsu, R. and Mori, A. 1991. Free Radical Biol. Med., 11:379-383.
- Osato, J.A., Santiago, L.A., Remo, G. et al. 1993. Life Sci., 53:1383-1389.
- Santiago, L.A., Osato, J.A. and Mori, A. 1992. Neurosciences, 18:189-194.
- Santiago, L.A. and Mori, A. 1993. Arch. Biochem. & Biophys. 306:16-21.
- Webman, E.J., Edlin, G.and Mower, H.F. 1989. Int. J. Radiat. Biol. 55:347-351.
- Osato, J.A., Cuadra, M., Santiago, L.A., Mori, A. and Horitsu, H. Proc. On Int. Conference on Bioradicals Detected by ESR Spectroscopy (in press).
Key words: free radical scavenger, antioxidant, Runn-runn