| Title | BIO-NORMALIZER AS A MODULATOR OF PHAGOCYTOSIS AND FREE RADICAL PRODUCTION BY MURINE INFLAMED NEUTROPHILS AND MACROPHAGES |
|---|---|
| Year | 1995 |
| Author | J.A. Osato, Igor B. Afanas’ev, Zinaida P. Cheremisina, Tatjana B. Suslova, Natalia E. Abramova, Elena V. Mikhalchik, Irina B. Deeva, Librado Santiago, and Ljudmila G. Korkina |
| Publisher | Physical, Chemical, Biological and Medicine |
BIO-NORMALIZER AS A MODULATOR OF PHAGOCYTOSIS AND FREE RADICAL PRODUCTION BY MURINE INFLAMED NEUTROPHILS AND MACROPHAGES
J.A. Osato1,2, Igor B. Afanas’ev3, Zinaida P. Cheremisina4, Tatjana B. Suslova4, Natalia E. Abramova4, Elena V. Mikhalchik4, Irina B. Deeva4, Librado Santiago1,5 and Ljudmila G. Korkina4
1 Osato Research Institute, Japan
2 The United Grade School of Agricultural Science, Gifu University, Japan
3 Vitamin Research Institute, Moscow, Russia
4 Russian Institute for Pediatric Hematology, Moscow, Russia
5 Department of Neuroscience, Okayama University, Medical School, Japan
ABSTRACT
In the animal model of aseptic inflammation, Bio-Normalizer (BN), a natural Japanese health food known as an agent possessing preventive and therapeutic activities in some pathologies such as tumors, inflammation, immunodeficiency, etc. was tested. The main aim of this work was to study the in vivo BN effects on phagocyte migration to inflammatory site, phagocytosis, and the organism’s free radical status. It was found that BN exhibited diverse effects on inflamed neutrophils and macrophages suppressing the neutrophil functions (migration and oxygen radical production), and activating macrophage migration, phagocytosis and superoxide release. BN also activated macrophage NADPH oxidase at the earlier stages of inflammation (1-3 days) and inhibited it at the end of one-week period. BN enhanced the SOD activity in inflamed macrophages but it did not affect that in inflamed neutrophils, blood erythrocytes and plasma and slightly decreased superoxide release from the blood erythrocytes induced by the prooxidant quinone menadione.
Our results suggest that BN is an effective modulator of oxidative stress associated with inflammation having a capacity to restore pro-/antioxidant balance. It was also proposed that the antitumor capacity of BN may be a consequence of macrophage activation via an interferon-g mechanism of the adaptation to oxidative stress.
Key words: Bio-Normalizer, Marcophags, Neutrophils, Oxygen Radicals, Superoxide
Bio-Normalizer (BN), a natural Japanese health supplementation prepared by the fermentation of Carica papaya, and containing yeast, glucose, amino acids and other bioactive substances, is thought to manifest preventive and therapeutic effects on numerous pathological states including tumors, allergy, inflammation, immunodeficiency, etc. (1). Until now, molecular and cellular basis for these activities remains obscure. In the most above-mentioned pathologies, the changes in the prooxidant/antioxidant balance are regarded as an important cause of their development. It was therefore proposed that therapeutic action of BN may be a consequence of its antioxidant properties. Indeed, it has been shown that BN is a hydroxyl radical scavenger and an inhibitor of iron-dependent lipid peroxidation (2). It was also proposed that glucose, as one of BN constituents is mostly responsible for BN antioxidant capacity (3).
We have shown recently (4) that the effects of BN on free radical processes are more complicated than it had been admitted earlier. Thus, although BN exhibited free radical-scavenging properties in various in vitro systems such as the Fenton reaction, xanthine-xanthine oxidase, or horse radish oxidase, it was able at the same time to stimulate or inhibit oxygen radical production by macrophages and PMN leukocytes, depending on the nature of the stimuli and the chemiluminiscence amplifiers applied.
The last findings seem to be especially important as PMN leukocytes are an essential factor of evolutionary developed host defense system against microorganism invasion (5). However, these cells also play a significant role in the host tissue damage during noninfectious inflammatory reactions (6). Upon the activation with various inflammatory agents, PMNs are accumulated within inflammation loci and release specific mediators including active oxygen species, proteolytic enzymes, the products of arachidonate oxidative metabolism, etc. All these substances may maintain and increase inflammatory lesions (7).
It is well established that mononuclear phagocytes are highly potent to kill microorganisms (8) and to inhibit tumor cell growth (9). However, their microbicidal and tumoricidal activities are apparently regulated by different ways. In response to inflammatory signal, blood monocytes migrate into the tissues and are converted there into macrophages, which are more responsive to the stimuli than resident macrophages (10). On the other hand, they may develop macrophage-mediated tumor cytotoxicity upon the stimulation with interferon-g, bacterial or yeast lipopolysaccharides (11). For example, there is a growing number of evidence that yeast polysaccharides possess a capacity of increasing host defense against bacteria, viruses, and tumors mainly by activating macrophages (12).
Thus it seems very important to study the in vivo effects of BN, which contains both the antioxidants and the potential activators of PMN and macrophage functions such as cell migration, phagocytosis, and oxygen radical production. Aseptic inflammation and subsequent cell influx into the peritoneum were induced by dextran sulfate injection to the mice. It was found that BN is a very potent inhibitor of PMNs accumulation and functions at inflammation site and, at the same time, is a promoter of macrophage activity. It was therefore concluded that BN exhibits well-compensated pro/antioxidant activity.
MATERIAL AND METHODS
Chemicals
BN is commercially produced by Sun-O International Inc., Gifu, Japan. Its partly soluble white sweet granules are prepared by the fermentation of Carica papaya and tropical herbal plants. The granules contain yeast (1-5 x 105 yeast cells/g), glucose (more than 2%), vitamins B and C, and papain proteases. Ferricytochrome c (type VIII), NADPH, lucigenin, luminol, menadione, zymosan A, latex (1 mm), PMA, epinephrine, Ficoll-Paque, Monoprep, bovine serum albumin, HBSS, phosphate buffer, and bovine CuZnSOD (EC 1.15.1.1) were purchased from Sigma Chem. Co. (St. Louis, Mo.). Dextran sulfate and sodium metrizoate were from Pharmacia, Sweden.
Animals
7-8 weeks old BalB/C mice weighing 18-20 g were used in the experiments.
In vivo experiments
Mice received intraperitoneal injection of 0.1 mL sterile physiological saline (Group 1, control, 6 animals) or 12.5 mg BN saline suspension (Group 2, 10 animals). Peritoneal inflammation was induced by the injection of 0.5 mL 0.06% dextran sulfate solution (Group 3, 8 animals and Group 4, 11 animals). 24 hours later, 0.5 mL BN (12.5 mg) saline suspension was injected to the group 4 mice. The mice were sacrificed and examined on the 1,2,3 and 8th day after the beginning of the experiment. Peritoneal exudate was collected by peritoneal rinsing with 2 mL saline solution. A total number of the cells collected from the peritoneal cavity was calculated by staining a 100 mL aliquot with 0.2% trypan blue and counting in a Goryaev chamber. Differential cell counting was performed in smears using Giemza staining.
Phagocytosis assay
A peritoneal cell suspension (0.5 mL) containing 2 x 106 cells was placed on the glass coverslips in 24 well plates (Costar, Cambridge, MA). Then, 0.5 mL (1 mg/mL) suspension of an opsonized zymosan was added to cell culture, and the samples were incubated at 37oC for 30 min. The coverslips were fixed with methanol and stained with Giemza. The number of the particles ingested by no less than 300 macrophages per coverslip was calculated by direct visual enumeration with a light microscope (x900). Upon the examination, the two parameters were recorded: the percentage of phagocytes in a cell population and the phagocytosing index. (A phagocyte has been defined as the macrophage ingested at least one zymosan particle). The percentage of phagocytes was calculated by dividing the number of phagocytes by the total number of macrophages counted and multiplying by 100. Phagocytosing index was calculated by dividing the number of particles ingested by the number of phagocytes (13).
Neutrophil and macrophage preparation
Mouse neutrophils and macrophages were prepared by density gradient centrifugation (14), using Ficoll-Hypaque (d=1.119) for neutrophil isolation and Monoprep (d=1.077) for mononuclear cell isolation. Cells were collected and washed twice with Ca, Mg-free HBSS and finally resuspended in minimal essential medium (MEM) at appropriate concentrations.
Erythrocyte isolation and plasma preparation
The blood of mice was collected in heparinized tubes (10 IU/mL). The whole blood from each mouse group was pooled and used for erythrocyte and plasma preparation. 2 mL blood sample was centrifuged at 150 x g for 5 min, erythrocyte pellet was collected and washed twice with a large volume of phosphate buffer. Washed cells were adjusted by 2% hematocrit with the same buffer. Supernatant after first centrifugation was transferred into other tube, and white blood cells were sedimented at 400 x g for 10 min. Top plasma layer was collected and stored at 20oC until analysis.
Superoxide production by erythrocytes
Spontaneous and menadione-induced superoxide production by erythrocytes was determined by SOD-inhibitable cytochrome c reduction (15). 20 mL erythrocyte suspension, 50 mM of cytochrome c were incubated in 0.1 M K-phosphate buffer (2 ml) with or without menadione (10 mM) at room temperature for 60 min. After centrifugation, the light absorbance of clear supernatant was measured at 542, 550, and 557 nm (Beckman DU 600 spectrophotometer). To eliminate possible hemoglobin absorbance, the amount of reduced cytochrome c was calculated by the following equation (15):
cyt c=47.6 A550 – 9.8A557 – 31.8A542
where the extinction coefficients of reduced and oxidized cytochrome c and oxyhemoglobin were taken into account. Data were presented as nmol O2–/60min/1010 erythrocytes. Each measurement was performed in triplicates, and the results were expressed as a mean ±SD.
Macrophage NADPH oxidase activity
The NADPH oxidase activity of macrophages was determined as it was described by Bellavite et. al. (16). Aliquot of 107 cells were suspended in HBSS (1 mL) together with 50 mM cytochrome c and 2 mM NaN3 (to prevent cyt. c reoxidation by cytochrome c oxidase) and placed at 37oC in the 1 cm light path polysterene cuvette supplied with a stirrer of a LKB “Ultraspec” spectrophotometer. Then, light absorbance was continuously recorded at 550 nm. The reaction was started by the addition of 10 ng/mL PMA, stopped at the maximal velocity by 0.05% Triton 100, and restored 1 min later by the addition of NADPH at appropriate concentrations.
Production of active oxygen species by neutrophils and macrophages
The production of active oxygen species peritoneal phagocytes was measured by the chemiluminiscence (CL) method using lucigenin as a specific CL probe for the detection of superoxide ion (17) and luminol as a CL probe for the detection of active oxygen species forming during hydrogen peroxide decomposition (18). In these experiments, 5×105 cells were mixed in polystyrene CL tubes with 0.9 mL HBSS containing 50 mM luminol or lucigenin. The tubes were placed in the CL unit of a LKB model 1251 luminometer (Sweden) equipped with minicomputer for controlling experimental procedures and calculation and expression of the obtained data. Cell-containing mixture was incubated at 37oC and continuous stirring for 5 min. During this time CL intensity (spontaneous CL) was registered continuously. Then, 50 mL of an activator solution (opsonized zymosan, latex particles, or PMA) was automatically added through a dispenser and the maximal intensity of CL response to the activator was measured (stimulated CL). Results were expressed in arbitrary CL units.
SOD activity in the whole blood, serum, isolated neutrophils and macrophages
Superoxide dismutase (SOD) activity was determined by the adrenaline method (19), in which the rate of superoxide production was measured by lucigenin-amplified CL. Heparinized blood (0.2 mL) or cell suspension (0.4 mL) was lyzed with ice-cold water. Lysates were added to an equal volume of ethanol-chloroform mixture (1:1 v/v) and centrifuged at 1500 x g for 30 min. Protein content in supernatant was determined by the Lowry method (20). To measure the total SOD activity, 50 mL of top clear supernatant or 50 mL of plasma were added to carbonate buffer (pH 10.2, a total volume of 900 mL) containing EDTA (100 mM) and lucigenin (100 mM) in a CL polystyrene cuvette, and the level of basal CL was registered continuously. Reaction was started by adding 50 mL adrenaline (100 mM) through the dispenser. The CL light sum for 5 min was recorded and compared with that of a control sample (50 mL of water:ethanol:chloroform solution (2:1:1 v/v/v)). The total SOD activity was calculated from calibration curve using commercial SOD as a standard and expressed as ng/mg protein (for macrophage, neutrophil and plasma SOD) and as mg/mg protein (for the whole blood).
Statistical analysis
Results are presented as mean ±SD for a given number of experiments. Differences were analyzed using the Student’s t-test, the level of significance being set at P<0.05.
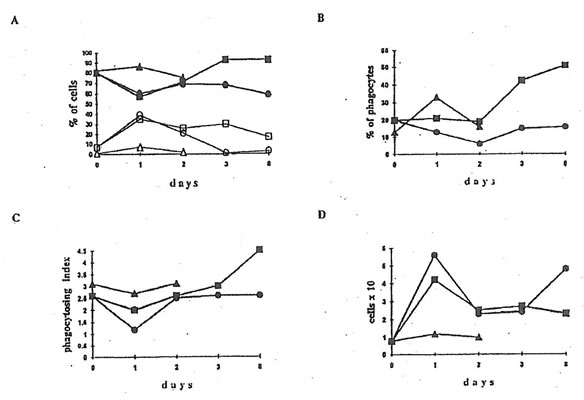
Fig.1. The effect administration on the functions of phagocytosing cells. 0.5 ml (1 mg/ml) of an opsonized zymosan suspension was added to 0.5 ml of a peritoneal cell suspension containing 2×106 cells, and the samples were incubated at 37oC for 30 min. The number of ingested particles was calculated by direct visual enumeration.
- Recruitment of Phagocytes. q and m are the points corresponding to the neutrophils from dextran-injected mice without and after BN application; ∆ are the points for the neutrophils from healthy mice after BN application. s and n are the points corresponding to the macrophages from dextran-injected mice without and after BN application; l are the points for the macrophages from healthy mice after BN application.
- Percentage of phagocytosis. Symbols are the same as for macrophages in Fig.1A.
- Phagocytosing index. Symbols are the same as for macrophages in Fig.1A.
- Recovery of cells in the murine peritoneal cavity. Symbols are the same as for macrophages in Fig.1A.
RESULTS
BN effects on cell influx and phagocytosis
The injection of dextran sulfate sharply stimulated the recovery of cells in the mouse peritoneal cavity (Fig.1D). BN administration lowered cell recovery to a normal value 4×106 cells per a mouse on 8th day of the experiment. The recruitment of neutrophils achieved a maximal 35% yield already the next day after the dextran injection with subsequent slow decrease to 18% on the 8th day (Fig.1A). The amount of peritoneal macrophages dropped down to 60% on the first day, then enhanced up to about 70% on the second day, and after that remained constant. The administration of BN drastically decreased the amount of neutrophils in the peritoneal cavity lowering their content almost to zero on the second day after BN application; at the same time the amount of macrophages increased by 93% (Fig.1A). Application of BN to the dextran-injected mice enhanced considerably the percentage of phagocytosis in comparison with both control and dextran-injected animals (Fig.1B). Phagocytosing index dropped from 26% to 13% on the first day after dextran injecting, however, its initial value was restored on the second day. The administration of BN enhanced the phagocytosing index by 45% on the 8th day (Fig.1C).
Effects of BN on cell chemiluminiscence
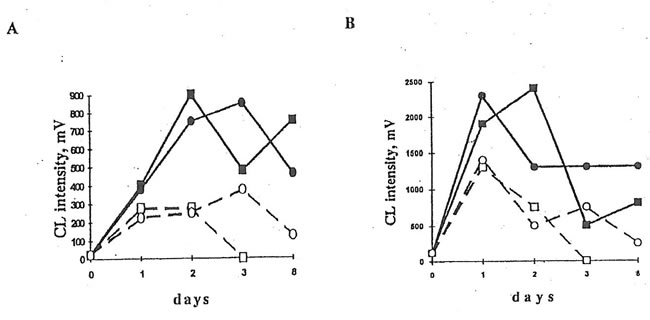
Injection of dextran sulfate to the mice promoted luminol- and lucigenin-amplified CL in the cells of peritoneal exudate stimulated with opsonized zymosan, latex particles, or PMA, maximal CL values being achieved on the first and second days after dextran injection. BN administration to the mice resulted in the significant changes in luminol- and lucigenin-amplified CL produced by latex- and PMA-stimulated peritoneal cells. (BN did not affect CL intensities in the case of zymosan stimulation). As it is seen in Figs. 1 and 2, BN affected differently macrophage luminol- and lucigenin-amplified CL. Thus, at the end of experiment when neutrophil-produced CL was close to zero, lucigenin-amplified CL of PMA- and latex-stimulated macrophages from the Group 4 mice was enhanced (Figs. 2A and 3B) while their luminol-amplified CL was inhibited (Figs. 2B and 3A). BN administration also shortened (at least in the case of PMA-stimulation, Fig. 2) the number of days when neutrophilic CL was observed.
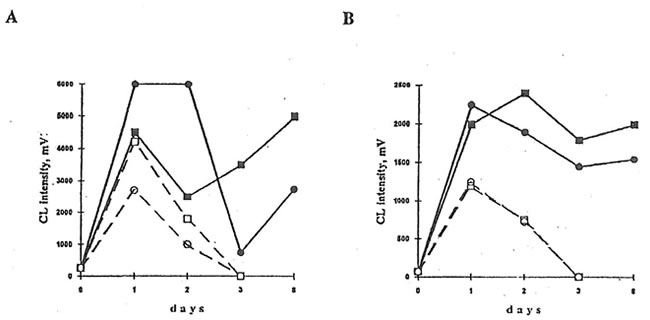
Furthermore, we studied the effects of BN on neutrophils and macrophages of healhy animals (Group 2). It was found (Fig. 4) that BN administration sharply increased (by 200-400%) both luminol- and lucigenin-amplified CL produced by non-stimulated and PMA-stimulated macrophages and inhibited neutrophilic CL by 25-75%. Similar results were obtained upon stimulation with latex particles and opsonized zymosan (data not shown).
NADPH oxidase activity of peritoneal macrophages
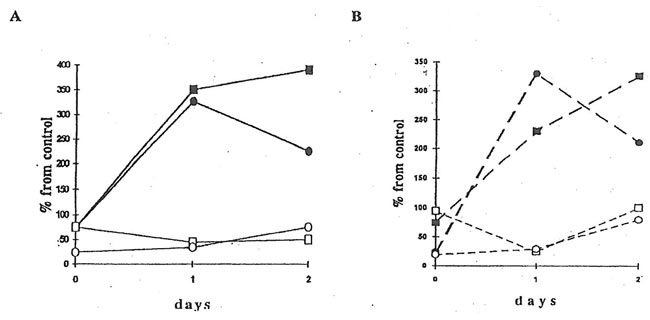
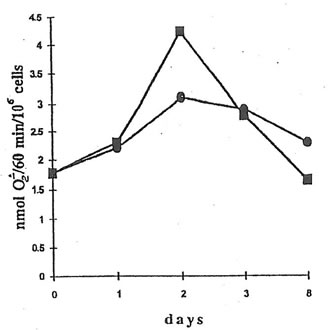
NADPH oxidase activity of peritoneal macrophages from the mice injected with dextran sulfate (Group 3) changed from 1.8 nmol O2-/min/106 cells to 3.1 nmol O2-/min/106 cells over the 8 days period (Fig.5). The administration of BN to the Group 4 mice enhanced NADPH oxidase activity by about 1.4 times on the second day (the first day after BN application), but to the end of the experiment, NADPH oxidase activity became even lower than that of macrophages from the mice without BN application (Group 3).
2. The Effects of BN administration to dextran-injected mice on luminol- and lucigenin-amplified CL produced by PMA-stimulated peritoneal macrophages and neutrophils. 5×105 cells were mixed with 0.9 mL HBSS containing 50 mM luminol or lucigenin, and cell-containing mixture was incubated at 37oC and continuous stirring for 5 min in the CL unit of a luminometer. During this time the intensity of spontaneous CL was recorded. Then, 50 ml of a PMA solution was added and a maximal intensity of CL response was measured.
A. Lucigenin-amplified CL. B. Luminol-amplified CL. n and l are the points corresponding to CL intensities produced by the inflamed macrophages without and after BN application. qand m are the same for neutrophils.
Fig.3. Effects of BN administration to dextran-injected mice on luminol- and lucigenin-amplified CL produced by latex stimulated peritoneal macrophages and neutrophils. Experimental conditions and symbols are the same as in Fig.2.
A. Luminol-amplified CL. B. Lucigenin-amplified CL
Fig.4. Effects of BN administration to healthy mice on luminol- and lucigenin-amplified CL produced by isolated peritoneal macrophages and neutrophils. Neutrophil and macrophages were prepared by density gradient centrifugation using Ficoll-Hypaque (d=1.119) for neutrophil isolation and Monoprep (d=1.077) for mononuclear cell isolation. CL experiments were carried out as shown in the legend to Figure 2. Symbols are the same as in Figs. 2 and 3.
A. Spontaneous luminol-amplified CL B. PMA-stimulated lucigenin-amplified CL
Fig. 5. NADPH oxidase activity of peritoneal macrophages from dextran-injected mice. An aliquot of 107 cells was suspended in HBSS (1 mL) together with 30 mM cytochrome c and 2 mM NaN3 and placed into the cuvette of a spectrophotometer at 37oC. The reaction was started by the addition of 10 ng/mL PMA, stopped at the maximal rate by 0.05% Triton, and restored 1 min later by the addition of NADPH. During the reaction, light absorbance was continuously recorded at 550 nm. l and n are the points corresponding to NADPH activity of macrophages from the mice without and with BN application.
Superoxide release by erythrocytes
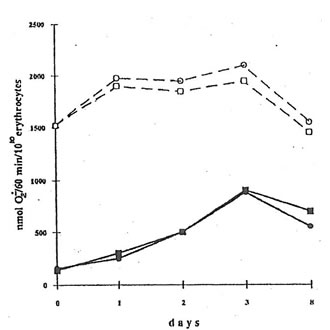
The injection of mice with dextran sulfate sharply increased spontaneous superoxide release (measured as a SOD-inhibitable cytochrome c reduction) from erythrocytes. Thus, while the rate of superoxide release by the erythrocytes of the mice from control Group 1 was equal to 120-60 nmol/60 min/1010 cells, that from dextran-injected mice (Group 2) was 900 nmol/60 min/1010 cells on the second day after BN application (Fig.6). Contrary to that, menadione-induced superoxide release by erythrocytes from control and dextran-injected animals was practically the same (about 1500-2100 nmol/60 min/1010 cells). BN application seems do not affect both spontaneous and menadione-induced superoxide release by erythrocytes from dextran-injected mice, although there was some decrease in menadione-induced superoxide release after BN application (Fig.6).
Fig. 6. Superoxide release by erythrocytes. 20 mL of an erythrocyte suspension, 50 mM cytochrome c were incubated in a 0.1 m K-phosphate buffer (2 mL) with or without menadione (10 mM) at room temperature for 60 min. After centrifugation, the light absorbance of supernatant was measured at 542, 550, and 557 nm. l and n are the points corresponding to the spontaneous superoxide release by the erythrocytes from dextran-injected mice without and after BN administration. m and q are the same for the menadione-induced superoxide release.
SOD activity in the whole blood, serum, peritoneal macrophages, and neutrophils
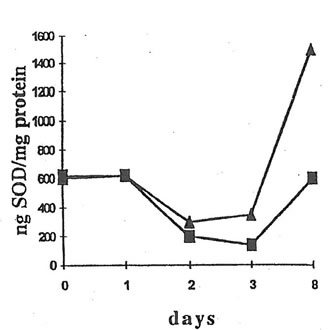
There was no change in the SOD activity in the whole blood and serum after BN administration to dextran-injected mice (data not shown). However, significant increase in SOD activity was observed in peritoneal macrophages on 7th day after BN application (Fig.7). Again, BN did not affect the SOD activity of peritoneal neutrophils (data not shown).
Fig. 7. SOD Activity in peritoneal macrophages. A macrophage suspension (0.4 mL) was lyzed with ice-cold water, lysates were added to an equal volume of an ethanol-chloroform mixture (1:1 v/v) and centrifuged at 1500 x g for 30 min. Then, 50 mL of top clear supernatant was added to carbonate buffer (pH 10.2) containing EDTA (100 mM) and lucigenin (100 mM), and the basal CL intensity was measured. The reaction was started by adding 50 mL of an adrenaline solution (100 M), and a CL light sum for 5 min was measured. n and s are the points corresponding to the SOD activity of macrophages from dextran-injected mice without and after BN administration
DISCUSSION
It is known that in response to inflammatory signal, blood monocyte migrate into the tissue and is converted there in the macrophages with much greater responsibility to the stimuli than the residual ones. These recruited prestimulated macrophages may also develop macrophage-mediated tumor cytotoxicity under the action of interferon-g or bacterial lipopolysaccharide (11). Furthermore, there is a growing number of evidence that yeast polysaccharides have the capacity of increasing host defense against bacteria, viruses, and tumors mainly by activating the macrophages. For example, it has been shown (21,22) that tumor cytotoxicity of some yeast components is a consequence of enhanced macrophage recruitment and functioning (phagocytosis, locomotion, adhesion, etc.) The macrophages activated with yeast polysaccharides interact with other immune cells, first of all, T-lymphocytes. As a result of this interaction, T-lymphocytes are able to produce interferon-g (23), thus completing the cycle of macrophage stimulation through specific receptors. Our present results show that BN can interfere with the stimulation of peritoneal macrophages and neutrophils, enhancing oxygen radical production by macrophages activated with particulate and soluble stimuli and diminishing the amount of oxygen radicals produced by neutrophils under the similar conditions. We suggest that the stimulating effect of BN on phagocytes may be a consequence of its ability to trigger the interferon-g formation by T-lymphocytes (as it has been shown for yeast polysaccharides (23)). Interferon-g may in its turn stimulate chemiluminiscence and hydroxyl radical production by macrophages (24). On the other hand, the antioxidant properties of BN showed by us (4) and other authors (2,3) are apparently responsible for the inhibition of neutrophilic oxygen radical production.
BN administration to mice affected weakly superoxide release by erythrocytes. Thus, BN is apparently ineffective in protection of erythrocytes from oxidative stress-induced damage.
We also found that BN stimulated the induction of SOD in macrophages and did not in neutrophils, protecting only the ones from damaging action of oxygen radicals released. It is well known that severe oxidative stress plays an important role in the pathogenesis of many diseases and is likely to contribute directly to the tumor initiation and promotion (25). On the other hand, moderate oxidative stress is responsible for the rapid induction of numerous gene products which are thought to serve as adaptogenes to the oxidative stress. Among these adaptogenes are SOD’s, catalase, DNA repair enzymes, etc. (25). Therefore, SOD is defined as a very important endogenous antitumor agent (26).
Our findings show that BN can be regarded as a new pharmacological agent possessing with double effects: on the other hand, BN is able to participate in the antioxidant defense against PMN leukocytes damaging capacity at inflammatory loci, and on the other hand, BN stimulates phagocytosing activity, oxygen radical production, and perhaps some other functions of mononuclear macrophages, which are likely major cells responsible for the mammalian antitumor immunity.
REFERENCES
- Santiago, L.A., Osato, J.A., Hiramatsu, M., Edamatsu, R., and A. Mori. 1991. Free Radical Scavenging Action of Bio-Catalyzer a.r No.11 (Bio-Normalizer) and its By-product. Free Rad. Biol. Med. 11:379-383.
- Santiago, L.A., Osato, J.A., Liu, J., and A. Mori. 1993. Age-Related Increase in Superoxide Dismutase and Thiobarbituric Acid-Reactive Substances: Effect of Bio-Catalyzer in Aged Rat Brain. Neurochem. Res. 18:711-717.
- Santiago, L.A., Osato, J., and A. Mori. 1992. Stability of the Hydroxyl Radical Scavenging Components of the Health Food “Bio-Normalizer”. Med.Sci.Res. 20:27-28.
- Osato, J.A., Afanas’ev, I.B., Cheremisina, Z.P., Suslova, T.B., Abramova, N.E., Mikhalchik, E.V., Deeva, I.B., Horitsu, H., Santiago, L.A., and L.G. Korkina. 1994. Effects of Bio-Normalizer on Free Radical Production by Human Blood Erythrocytes, Neutrophils, and Rat Peritoneal Macrophages. Submitted to publication.
- Forman, H.J., and N.J. Tomas. 1986. Oxidant Production and Bactericidal Activity of Phagocytes. Ann. Rev. Physiol. 48:669-680.
- Taffet, S., and S.W. Russel. 1981. Macrophage-Mediated Tumor Cell Killing: Regulation of Expression of Cytolytic Activity by Prostaglandin E. J. Immunol. 126:424-429.
- Nathah, C. F., Pruckner, L., Silverstein, S.C., and Z. Cohr. 1979. Extracellular Cytolysis by Activated Macrophages and Granulocytes. J. Exp. Med. 149:84-113.
- Klebanoff, S.J. 1980. Oxygen Metabolism and the Toxic Properties of Phagocytes. Ann. Intern. Med. 93:480-486.
- Cameron, P.J., and W.H. Churchill. 1979. Cytotoxicity of Human Macrophages for Tumor Cells. Enhancement of Human Lymphocytes Mediators. J. Clin. Invest. 64:977-984.
- Schulz, R.M., Papamatheakis, J.D. and M.A. Chirigos. 1977. Interferon: An Inducer of Macrophage Activation by Polyanions. Science. 197:674-675.
- Uhing, R.J., and D.O. Adams. 1989. Molecular Events in the Activation of Murine Macrophages. Agents and Actions. 26:9-14.
- Koerner, T.J., D.O., and T.A. Hamilton. 1987. Regulation of Tumor Necrosis Factor (TNF) Expression: Interferon-g Enhances the Accumulation of mRNA for TNF Induced by Lipopolysaccharide in Murine Peritoneal Macrophages. Cell Immunol. 109:437-443.
- Grasso, R.J., Klein, T.W., and W.R. Benjamin. 1981. Inhibition of Yeast Phagocytosis and Cell Spreading by Glucocorticoids in Cultures of Resident Murine Peritoneal Macrophages. J. Immunopharmacol. 3:171-175.
- Lindena, J., and Purkhart, H. 1988. Separation and Chemiluminiscence of Human, Canine and Rat Polymorphonuclear Cells. J. Immun. Methods. 115:141-148.
- Grinberg, L.N., Shalev, O., Goldfarb, A., and E.A. Rachmilewitz. 1992. Primaquine-Induced Superoxide Production by b-Thalassemic Red Blood Cells. BioChim. Biophys. Acta. 1139:248-250.
- Bellavite, P., Jone, O.T.C., Cross, A.R. Papini, E., and F. Rossi. 1984. Composition of Partially Purified NADPH Oxidase from Pig Neutrophils. Biochem. J. 223:639-648.
- Gyllenhammar, H. 1987. Lucigenin Chemiluminiscence in the Assessment of Neutrophil Superoxide Production. J. Immunol. Methods. 97:209-214.
- Allen R.C., and L.D. Loose. 1976. Phagocytic Activation of a Luminol Dependent Chemiluminiscence in Rabbit Peritoneal Macrophages. Biochem. Biophys. Res. Commun. 69:245-254.
- Misra, H., and I. Fridovich. 1972. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for SOD. J. Biol. Chem. 247:3170-3175.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., and R.J. Randall. 1951. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 193:265-275.
- Fantone, J.C., and P.A. Ward. 1982. Role of Oxygen-Derived Free Radicals and Metabolites in Leukocyte-Dependent Inflammatory Reactions. Am. J. Pathol. 107:397-418.
- Mayer, P., and J. Drews. 1980. The Effect of Protein-Bound Polysaccharide from Coriolus Versicolor on Immunological Parameters and Experimental Infections in Mice. Infection. 8:13-21.
- Adams, D.O., and T.A. Hamilton. 1988. Phagocytic Cells: Cytotoxic Activities of Macrophages. Inflammation: Basic Principles and Clinical Correlates. (Eds. Gallin, J.I.; Goldstein, I.M.; Snyderman, R.) Raven Press, New York. 471-492.
- Ito, M., Karmali, R., and M. Krim. 1985. Effect of Interferon on Chemiluminiscence and Hydroxyl Radical Production in Murine Macrophages Stimulated by PMA. Immunology. 56:533-541.
- Oberley, C.W., and G.R. Buettner. 1979. Role of Superoxide Dismutase in Cancer: A Review. Cancer Res. 39:1140-1149.
- Sun, Y. 1990. Free Radicals, Antioxidant Enzymes, and Carcinogenesis. Free Rad. Biol. Med. 8:583-590.