| Title | ABSTINENCE-INDUCED OXIDATIVE STRESS IN MODERATE DRINKERS IS IMPROVED BY BIO-NORMALIZER |
|---|---|
| Year | 1997 |
| Author | Marotta F., Reizakovic I., Safran P., Barbi G. |
| Publisher | The International Journal of Hepatogastroenterology |
Abstinence-Induced Oxidative StreSS in Moderate Drinkers is Improved by Bio-Normalizer
Marotta F., *Reizakovic I., + Safran P., Barbi G.
Gastroenterology Services, S. Anna Hospital, Como, Italy
* Liver Unit, Niguarda Hospital, Milano, Italy
+ Econum Lab., Villeneuve d’ Ascq, France
Corresponding author:
Francesco Marotta, MD, PhD
Via Pisanello, 4
20146 Milano
Italy
Tel/fax: +39-2-4077243
Abstract
The aim of this investigation was to study the oxidative phenomena which take place in the early recovery phase after alcohol withdrawal. Furthermore, the effects of a novel natural antioxidant, Bio-Normalizer (BN), in such a clinical setting was studied.
Forty-six alcoholics with moderate drinking habits (daily ethanol intake: >80 g to < 120g) were enrolled in the study, divided into two groups and given either a placebo or 9 g of BN by mouth every night for one week. The patients agreed to stop drinking alcohol, and daily blood sampling was obtained for routine tests and to check plasma and erythrocyte levels of MDA, SOD, GPX and the hydroperoxide level. The groups were comparable in terms of initial biochemical parameters.
BN prevented the early increase of plasma TBARS observed in the placebo group, enabling a near-to-normal level of plasma and erythrocyte MDA by the fourth day. BN also prevented the significant drop of erythrocyte GPX and the transient decrease of plasma SOD observed in the placebo group. Despite alcohol withdrawal, plasma lipid hydroperoxide remained significantly elevated in the placebo group, but this phenomenon was rapidly improved by BN.
To a significant extent, BN is able to prevent the free radical-mediated lipoperoxidative changes that occur soon after alcohol withdrawal, while fastening the recovery mechanisms.
Key Words: Alcohol Withdrawal; Antioxidant Status; Free Radicals; Bio-Normalizer
Abbreviations: Thiobarbituric Reactive Substances (TBARS), Superoxide Dismutase (SOD), Malonildealdehyde (MDA), Glutathione Peroxidase (GPX)
Introduction
Alcohol oxidation causes a wide range of metabolic and pathologic changes in the liver, and lipid peroxidation is an important mechanism which occurs during alcohol-induced liver damage (1-3). In particular, microsomal hydroxyl radical products in the liver during alcohol oxidation have been shown to increase the serum level of malonildialdehyde (MDA) (4). This is a stable product resulting from free radical-mediated peroxidation of polyunsaturated fatty acids of cell membranes (5). On the other hand, the concentration of antioxidants may be inadequate in alcohol-abusers, especially when highly reactive oxidant species are generated in large amounts (6). Chronic alcohol consumption enhances the activity of the microsomal ethanol oxidizing system. Furthermore, Lieber et al. (7) have also shown that such increased metabolic activity may persist independently of the constant presence of alcohol intake. Thus, this phenomenon would perpetuate a pro-oxidative condition in the body of alcoholics during abstinence.
The purpose of this study was to address the issue of the oxidative phenomena which take place in the early recovery phase of alcohol withdrawal in alcoholics, while testing a novel, natural Japanese health food, Bio-Normalizer, which is endowed with potent free radical-scavenging properties, as ascertained by recent studies (8-10).
MATERIALS AND METHOD
Patients
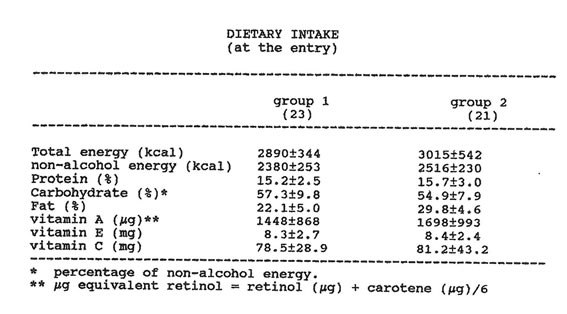
Forty-six alcoholics (34 males and 12 females; age range: 34-55), with a moderate drinking habit (>80 g to <120 g ethanol daily for at least 3 years) were enrolled in the study. None of the patients had clinical or biochemical evidence of acute liver disease, and they were all free of HBV and HCV infection. All of the patients underwent a careful personal interview to establish ethanol consumption and drinking behavior. A detailed diet-history method was carried out (Table 1), and nutritional intake was calculated by using a standardized food-composition table. None of the patients were febrile or malnourished.
To enter the study, patients were selected by their willingness and practical compliance in abstaining from alcohol, at least throughout the study period. Their reliability was checked by blood ethanol measurement (spectrophotometric method based on NAD reduction) as well as by other routine liver tests. The patients were also instructed not to perform any strenuous physical exercise during the observation period.
The patients were randomly divided into two groups (23 patients each), which were matched for gender, age, and nutritional status. On the day the patients stopped drinking alcohol, they were allocated into one of the following groups:
a) The no-treatment group, which served as the control group. To assure psychological compliance, for one week the patients were blindly give 9 g of flavoured sugar by mouth per night, which was devoid of antioxidants or vitamins.
b) The experimental group, which received Bio-Normalizer in the form of a whitish, sweet powder, 9g of which was taken every night by mouth for 1 week.
No vitamin supplementation was allowed during the study period. As healthy controls, 15 age- and gender-matched teetotaler subjects were also studied. The patients underwent daily follow-up for one week, which included clinical examinations, dietary interviews and biochemical tests, as outlined below.
Methods
Blood samples were taken using EDTA as an anticlotting agent and immediately refrigerated in ice. The plasma was separated by centrifugation at 4°C, and the leucocyte layer was promptly removed by aspiration.
Plasma TBARS were assayed by the method of Yagi (11) and modified by adding 0.01% butylated-hydroxytoluene to the coloring solution to avoid formation of TBA-reactive molecules during the analytic procedure. Tetra-ethoxy-propane served as the standard source of malonil-dialdehyde, which is a TBARS. All samples were processed in duplicate. The remaining erythrocytes were washed in %ml ice cold phosphate buffered saline with a pH of 7.4 and sedimented for five minutes at 1500 rpm. Aliquots of 0.5ml of the packed erythrocytes were added to 1 ml of 50% trichloroacetic acid. Malonildialdehyde in the erythrocyte extracts was assayed by HPLC analysis, according to Esterbauer et al. (12). Briefly, 0.2 ml of packed erythrocytes were thoroughly mixed with the same volume of ice cold acetonitrile in 1.5 ml Eppendorf tubes. Following 20 minutes of extraction in ice, the samples were centrifuged for one minute at 13,000 rpm, and 20 ml of supernatant was analyzed by HPLC (Lichrospher column, Merck, Darmstadt, Germany) with acetonitrile/30mM TRIS buffer, pH 7.4 (1:9 vol/vol) as the eluent. The effluent was monitored at a 270 nm wavelength, and the malonildialdehyde peak in the chromatogram was identified by comparison with that of free malonildialdehyde standard, which was freshly prepared by the hydrolysis of malonildialdehyde-bisdiacetal in 1% sulphuric acid solution. Glutathione peroxidase (GPX) and superoxide dismutase activities (SOD) of the plasma and erythrocytes were determined in a centrifugal analyzer (Hoffman La Roche, Switzerland), as described by Guemori et al. (13). Plasma lipid hydroperoxides, primarily phospholipid hydroperoxides, were estimated by hemoglobin catalysed oxidation of 10-N-methylcarmoyl-3,7-dimethylamino-10-H-phenothiazine after treatment with phospholipase D, according to Ohishi et al. (14). Cumene hyroperoxide was used as the standard. Plasma phospholipid was determined by an enzymatic kit (Boehringer Biochemia, Mannheim, Germany). The plasma content of alpha-tocopherol was measured by HPLC analysis, according to Burton et al. (15). Briefly, plasma aliquots (1 ml) were mixed with 1 ml of 100 mM sodium dodecylphosphate solution in water, 2 ml of absolute ethanol and 1 ml of n-heptane and shaken for one minute. Following 15 minutes of extraction in the dark, the heptane phase was separated by centrifugation, and 50 ml aliquots were used for the HPLC assay. Values were read by a fluorescence detector set at 296 nm excitation and 325 nm emission wavelength.
Statistical Analysis
Data were analyzed using the non-parametric Mann-Whitney U-test, and correlations were tested using the Kendall-Tau test for a non-parametric data.
Results
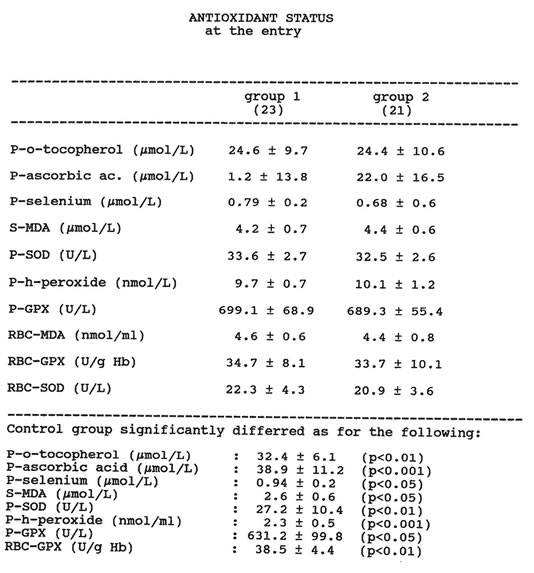
Two patients taking Bio-Normalizer were withdrawn from the study because one admitted drinking a few light alcohol beverages on the fifth observation day and another missed one blood check-up. The two groups of patients considered were comparable in terms of baseline routine liver function tests and smoking habits. The smokers were nearly 50% in both groups, and gamma-glutamyl-tanspeptidase was the most significant biochemical abnormality (over four-fold increase). The drinking habits were comparable in terms of pattern and quantity of alcohol consumption in both groups, daily wine intake being the most common form of alcohol intake. No statistical difference appeared between the groups as far as dietary components during the study period. In particular, regarding dietary vitamin intake, no significant difference was recorded between the two groups of alcoholics and the healthy control group. Table 2 shows the baseline data regarding the antioxidant status of the two groups of alcohol abusers. At the time of entry, as compared to the healthy controls, both groups showed significantly lower levels of plasma alpha-tocopherol, plasma ascorbic acid, plasma selenium and erythrocyte GPX. On the other hand, the levels of serum and erythrocyte MDA and the plasma levels of GPX, SOD, and lipid hydroperoxides were significantly higher in the alcoholics. No significant difference appeared between the two groups of patients in the initial status of oxidative stress. Routine liver tests, body weight and smoking habits were not affected by Bio-Normalizer treatment. However, as compared to the placebo group, the patients treated with Bio-Normalizer reported a trend of increased appetite, as ascertained by the specific dietary interview.
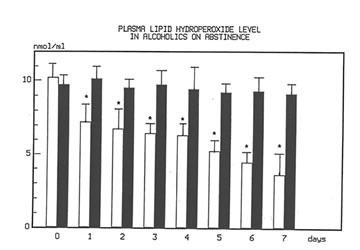
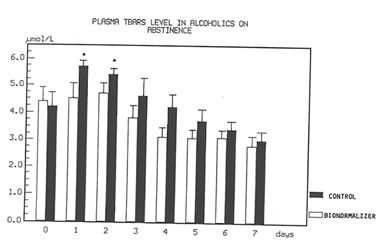
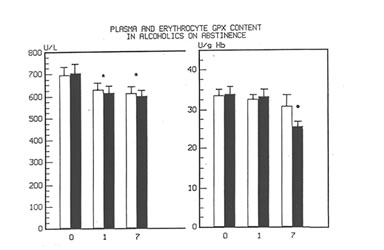
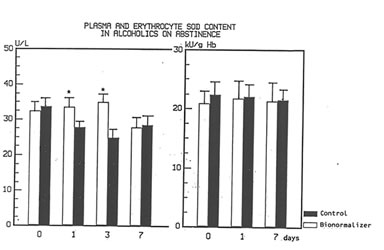
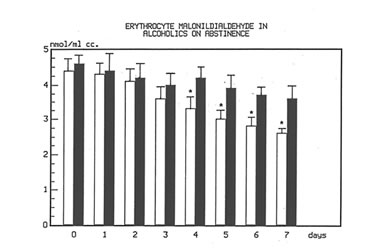
Figure 1 shows the effect of Bio-Normalizer on plasma TBARS. After alcohol cessation, a significant early increase (p<0.05) of TBARS was observed in patients under no treatment. This increase was self-limited by returning to normal values by the seventh day. However, concomitant supplementation with Bio-Normalizer prevented such a phenomenon (p<0.01) by enabling a steady state of plasma TBARS and a return to values comparable to healthy controls by the fourth day. From the erythrocyte MDA assay, it appeared that during the observation period, a decrease trend was observed in the placebo group. However, no significant change occurred. As compared to the pre-withdrawal values, this parameter in the Bio-Normalizer group showed a significant decrease from the fourth day (p<0.05) (Figure 2). Both the plasma GPX and erythrocyte GPX in both groups showed a decrease during the abstinence period, irrespective of the treatment. However, while at the end of the study period the erythrocyte GPX level in the placebo group was significantly lower that in the pre-withdrawal period (p<0.05), this did not occur in the Bio-Normalizer group (Figure 3). The SOD plasma level was significantly higher in both groups of drinkers, both at the time of entry and at the end of the study (p<0.05). This parameter showed a transient but significant decrease following alcohol withdrawal in the placebo group (p<0.05). Conversely, Bio-Normalizer brought about by an inverse slope, with three day observation levels significantly increased, as compared to the placebo group (p<0.05). Irrespective of the treatment, the erythrocyte SOD did not show any significant change over the abstinence period (Figure 4). The plasma lipid hydroperoxide level in alcoholics was significantly higher than in the controls (p<0.01), and no change occurred in the placebo group during the observation period. However, starting from the first observation-day, a significant decrease was recorded in the Bio-Normalizer (7.1±1.3 nmol/ml vs 9.8±0.9 nmol/ml, p<0.05) group, with near-to-normal levels at the end of the study (3.2±1.3 nmol/ml).
Discussion
Our baseline date supported the established knowledge of reduced serum levels of selenium, alpha-tocopherol and ascorbic acid in alcoholics. Furthermore, only a marginal recovery of these parameters was observed after one-week abstinence period, irrespective of the treatment. During the metabolism of alcohol to acetaldehyde and its further metabolic process by cytochrome P45k02E1, toxic free radical metabolites are generated. Such products trigger lipoperoxidative reactions which, in turn, are implicated in the pathogenesis of liver damage (16-18). Consistently high levels of cytochrome activity (up to ten-fold) as well as enhanced liver mitocondrial sensitivity to lipid peroxidation may persist for a while after the cessation of alcohol intake in cases of chronic exposure to ethanol (7), and particularly in the early withdrawal stage (19). However, there have been only scanty reports on the effect of alcohol withdrawal on the oxidative status (6). Indeed, by our closely monitored data, it appeared that early in the course of abstinence, oxidative stress indicators, such as plasma lipid hydroperoxide and erythrocyte and plasma MDA, in the placebo group maintained a level comparable to the pre-withdrawal period and were thus significantly higher than in the healthy controls. In particular, it was of interest to note that plasma MDA in this group showed a transient but significant further increase by the third observation day. Despite controversies which still exist in the literature, in accordance with Lecomte et. al. (6) erythrocyte SOD was unaffected by either alcohol ingestion or abstinence, irrespective of the treatment administered.
Plasma measurement of glutathione is an unreliable indicator of hepatic stores. Furthermore, chronic ethanol feeding accelerates the turnover of GSH. Additionally, the CU/ZN-SOD enzyme, which is the first line of defense against oxygen-derived free radicals, is rapidly induced following oxidative stress. However, such a tentatively compensatory measure might be insufficient, given the critical balance of the overall antioxidant system in alcoholics, something which also appeared in our patients. Accordingly, baseline and one-week observation levels of plasma SOD and gluthathione in both groups of drinkers was significantly higher than in the healthy controls. However, in the placebo group, a decreasing trend was observed ruing the first three days, with a statistically lower value on the third day. These data suggest that a pro-oxidative condition with avid consumption of SOD and glutathione still takes place once alcohol ingestion is stopped. On the other hand, the present investigation suggests a beneficial effect of oral administration of Bionormalizer, a natural free radical-scavenger, in the acute phase of recovery from oxidative stress following long-lasting moderate alcohol abuse. Eryhtrocytes in patients with alcohol-related liver disease may display an enhanced susceptibility to oxidative stress due to derangement of structural membrane lipids and to metabolic abnormalities. Accordingly, a reduced erythrocyte half-life has been shown in patients with moderate to severe liver cirrhosis. With Bionormalizer treatment, the erythrocytes were far less susceptible to peroxidative changes (significant reduction of erythrocyte MDA and partial sparing of erythrocyte GPX depletion). If the susceptibility of erythrocytes to peroxidative stress reflects that of other cells, and of hepatocytes in particular, then alcoholics would benefit from increased dietary supplementation of effective natural free radical-scavengers, such as Bio-Normalizer. The optimal protection of the cell against oxidative damage is likely to be a result of a complete and balance interrelationship between several antioxidant systems which lay on constitutive and newly synthetized forms.
In conclusion, while limitations have to be considered in the present study in terms of the number of patients and the period of observations, the modulatory effect exerted by Bio-Normalizer seems to be or worthwhile interest when taking into account that very recent experimental data have questioned the preventive effect of a-tocopherol on ethanol-induced hepatic lipoperoxidative changes by showing, on the contrary, that high doses promote concomitant oxidative reactions in the liver with undesirable metabolic consequences (20). The administration of doses of Bio-Normalizer up to three times those employed in the present study have not shown any such untoward effect (Marotta, F., unpublished data).
References
- Videla, L., Fernandez, Ugarte, G., et. al. Effect of acute ethanol intoxication on the content of reduced glutathione of the liver in relation to its lipoperoxidative capacity in the rat. FEBS Letter 1980; 111:6-10.
- Videla, L., Valenzuela, A. Alcohol ingestion, liver glutathione and lipoperoxidation: metabolic interrelations and pathological implications. Life Sci. 1982; 31:2395-2399.
- Lieber, C.S. Metabolism and metabolic effects of ethanol. Sem .Liv. Dis. 1981; 1:189-202.
- Esterbauer, H. Lipid peroxidation products: formation, chemical properties and biological activities. In: Poli G., Cheeseman K.H., Dianzani M.U. and Slater T.F. (eds.) Free Radicals in Liver Injury. IRL Press, Oxford 1985, pp. 29-47.
- Esterbauer, H., Schaur, R.J., Zollner, H. Chemistry and bio-chemistry of 4-hydroxynonenal, malonildialdehyde and related aldehydes. Free Radic. Biol. Med. 1991; 11-81-128.
- Lecomte, E., Herberth, B., Pirollet, P., Chancerelle Y., et. al. Effect of alcohol consumption on blood antioxidant nutrients and oxidative stress indicators. Am. J. Clin. Nutr. 1994; 60:255-261.
- Lieber, C.S. Biochemical and molecular basis of alcohol induced injury to liver and other tissues. N. Engl. J. Med. 1988; 319:169-1642.
- Santiago, L.A., Osato, J.A., Hiramatsu, M., et. al. Free radical scavenging action of Bio-catalyzer and its bioproducts. Free Radic. Biol. Med. 1991; 11:379-383.
- Haramaki, N., Marcocci, L., D’Anna, R., et. al. Bio-catalizer a-r No. 11 (Bio-Normalizer) supplementation: effect on oxidative stress to isolated rat hearts. Biochem. Molec. Biol. Internat. 1995; 36:1263-1268.
- Korkina, L., Osato, J.A., Chivilyeva, I., et. al. Radioprotective and antioxidant effect of zinc aspartate and Bio-Normalizer in children with acute myelo-and lympholeukemias. Nutrition 1995; 11:555-558.
- Yagi, K. Lipid peroxide and human diseases. Chem, Phys, Lipids 1987; 45:337-351.
- Esterbauer, H., Lang, J., Zadravec, S., et. al. Detection of malonidialdehyde by high-performance liquid chromatography. Meth. Enzymol. 1984; 105:319-325.
- Guemori, L., Artur, Y., Herbeth, B., et. al. Biological variability of superoxide dismutase, glutathione peroxidase and catalase in human blood. Clin. Chem. 1991; 37:1932-1937.
- Ohishi, N., Ohkawa, H., Miike, A., et. al. A new assay method for lipid peroxides using a methylene blue derivative. Biochem. Int. 1985; 10:205-211.
- Burton, G.W., Webb, A., Ingold, K.U. A mild, rapid and efficient method of lipid extraction for use in determining vitamin E lipid rations. Lipids 1985; 20:29-39.
- Nordmann, R., Ribiere, C., Rouach, H. Involvement of oxygen free radicals in the metabolism and toxicity of ethanol. In: Goedde HW, Agarwal DP (eds.), Genetics and alcoholism, Alan R. Liss Publ., New York, 1987 pp.201-213.
- Situnayake, R.D., Crump, B.J., Thurnham, D.I., et. al. Lipid peroxidation and hepatic antioxidants in alcoholic liver disease. GUT 1990; 31:1311-1317.
- Teare, J.P., Greenfield, S.M., Watson, D., et. al. Lipid peroxidation in rats chronically fed alcohol. GUT 1994;35:1644-1647.
- Rouach, H., Clement, M., Orfanelli, M.T., et. al. Hepatic lipid peroxidation and mithocondrial susceptibility to peroxidative attacks during ethanol inhalation and withdrawal. Biochim. Biophys. Acta 1983; 753:439-444.
- Boudry, S.C., Guo, S.X., and Adansa, J.D. Prevention of ethanol-induced changes in reactive oxygen parameters by a-tocopherol. Alcohol and Alcoholism 1996; 31:403-410.
Table 1 ANTIOXIDANT STATUS at the entry
Table II DIETARY INTAKE (at the entry)
(a) percentage of non-alcohol energy
(b) µg equivalent retinol = retinol (µg) + carotene (µg)/6
Fig 1
Time course changes of plasma lipid hydroperoxide level in alcoholics on abstinence with or without Bionormalzer treatment.
P.0.01 vs. control group
Fig 2
Time course of plasma TBARS in alcoholics with and without treatment with Bio-Normalizer while on abstinence.
*p<0.01 vs. Bionormalizer group.
Fig 3
Time course changes of plasma and erythrocyte GPX content in alcoholics on abstinence/ and with or without Bionormalizer treatment.
*p <0.05 vs. pre-withdrawal level,
• p <0.05 vs. Bionormalizer group.
Fig 4
Time course changes of plasma and erythrocyte SOD Level in alcoholics on abstinence and with or without Bionormalizer treatment.
*p< 0.05 vs. control group.
Fig 5
Figure 5. Time-course changes of plasma lipid hydroperoxide level in alcoholics on abstinence, with and without Bio-Normalizer treatment. * p<0.01 vs. control group.