| Title | Age-related increases in methylguanidine: Inhibitory effect of Bio-catalyzer in aged rat brain |
|---|---|
| Year | |
| Author | Librado A. Santiago, James Akira Osato, Rei Edamatsu, and Akitane Mori |
| Publisher |
Age-related increases in methylguanidine: Inhibitory effect of
Bio-catalyzer in aged rat brain
Librado A. Santiago, James Akira Osato1, Rei Edamatsu, and
Akitane Mori*
Department of Neuroscience, Institute of Molecular and Cellular Medicine, Okayama University Medical School, 2-5-1 Shikata-cho, Okayama 700, Japan; 1Sun-O International Inc., Gifu 500, Japan
Running title: Inhibitory action of Bio-catalyzer- on methylguanidine during ageing
*Address correspondence to:
Akitane Mori, M.D., Ph.D.
Chairman, Department of Neuroscience
Institute of Molecular and Cellular Medicine
Okayama University Medical School
2-5-1 Shikata-cho, Okayama 700, Japan
Tel: 0862-23-7151 (ext. 2640)
Fax: 086-2-32-2426
ABSTRACT
By HPLC-guanidino compound analyzer coupled with electrochemical detector using the phenanthrenequinone reaction, methylguanidine (MG) – a potent uremic toxin and a hydroxyl radical-mediated peroxidative of creatinine (CRN) – was found to increase with age in rat brain. Likewise, age-related increases were observed on creatinine (CRN) and -guanidinoacetic acid (GAA).
Oral administration of 0.1 g/kg body weight of Bio-catalyzer for 27 weeks on 89 week-old rats decreased significantly the elevated levels of MG and CRN in the brain – indicative of the antioxidant role of Bio-catalyzer in inhibiting hydroxyl radicalmediated synthesis of MG from CRN.
Keywords: methylguanidine, creatinine, guanidine, lipid peroxidation, hydroxyl radical, free radical scavenger/antioxidant, Bio-catalyzer.
INTRODUCTION
Methylguanidine (MG), a known potent uremic toxin1-2 is distributed, aside from kidney, in the rat heart3, erythrocyte3, intestine3, liver4, plasma3,4, and brains5-8. It is formed from creatinine (CRN) via reactive oxygen species (ROS) in particular the hydroxyl radical-generated Fenton reagents10,11. Free hemoglobin acting as a biological Fenton reagent12 and iron promoter forms hydroxyl radical. Since hydroxyl radical is implicated in various diseases and disorders, determination of MG as a marker of this radical could prove useful in studying the mechanism of these free radical related diseases and disorders.
The hydroxyl radical has been used as a marker in uremic and hemodialyzed patients14, rat hepatocytes15 and brain16-18. High levels of methylguanidine have been demonstrated to induce convulsion in cats and rabbits19 and epileptic seizures in mice20. No data however is available on the formation of MG and its derivatives during the ageing process. By high performance liquid chromatographic and fluorometrical analyses using the phenanthrenequinone (PQ) reaction 21,22, we determined MG and its precursor CRN9 and – guanidinoacetic acid (GAA) in adult and aged rat brain.
Bio-catalyzer, a commercial natural fermented health food supplement in Japan with proven scavenging action on free radicals and lipid peroxide in epileptic foci in rats23 indicative of its anti-epileptic property, increased the mitochondrial and cytosolic superoxide dismutase and decreased thiobarbituric acid-reactive substances in aged rat brain (unpublished report) – suggesting its anti-ageing property. Since free radicals are normal metabolic products of oxidative stress, we studied their effects and the effect of oral administration of Bio-catalyzer on these vital guanidino compounds in the brain of aged rats.
MATERIALS AND METHODS
Bio-catalyzer Bio-catalyzer α·r No. 11 (Bio-normalizer) is a white, sweet health food preparation produced by fermentation of Carica papaya Linn. (a Philippine medicinal plant) and other tropical herbal plants23; and is available commercially from Sun-O International Inc., Gifu, Japan.
Animals Male Sprague-Dawley rats from Clea Japan Inc., (Tokyo, Japan) aged 12 and 89 weeks were used. Thirty five aged rats (89 week-old) were housed in individual aluminum cages for 27 weeks (including 1-week acclimatization) at 20- 26oC, at humidity of 40-70%, ventilation of 15-25 times/h, light of 150-160 lux for 12 h/day from 7:00 to 19:00, noise < 60 phon, ammonia of < 20 ppm, air stream of 25 cm/sec and were maintained under strict sanitary conditions. The animals were allowed free access to water and a standard diet (CE-2 pellet supplied by Clea Japan, Inc., Tokyo). The rats were divided randomly into two groups; the control group consisting of 20 rats was orally administered with tap water boiled for 30 min while the second group of 15 rats was orally administered with 0.1 g/kg body weight (BW) of Bio-catalyzer for 27 consecutive weeks (189 days) at a volume dosage of 0.1 ml/100g animal weight. This dose approximates the recommended dose for humans which are 3-9 g/day. The administration was done from 14:00 to 17:00 after 2-3 h period of restricted food and water. The rats were sacrificed on the 27th week of Bio-catalyzer treatment by cervical decapitation and were immersed in dry ice, with the whole brain rapidly removed. The cortex was dissected on an ice plate24, frozen in liquid nitrogen and kept at 80°C until analysis.
Chemicals Guanidino standards such as guanidinoacetic acid (GAA), creatinine (CRN), guanidine (G) and methylguanidine (MG) were obtained from Wako Pure Chemical Ind. Ltd., Osaka; homoarginine (HArg), guanidinosuccinic acid (GSA) and guanidinopropionic acid (GPA) from Sigma Chemical Co., St. Louis, MO; arginine (Arg) from Katayama Chemical, Osaka; guanidinoethanesulfonate (GES) and guanidinobutyric acid (GBA) from Ono Pharmaceutical Co., Osaka; N-acetylarginine (NAA) from Eastman Kodak Co., NY; and guanidinoglutaric acid (GGA) was synthesized from glutamic acid and S-methylisothiourea25. Sodium hydroxide (NaOH), sodium citrate, sodium capric acid, boric acid 9,10- phenanthrenequinone (PQ), and N,N’-dimethylformamide (DMF) were purchased from Wako Pure Chemical, Osaka while hydrochloric acid (HCI) was from Nacalai Tesque, Inc., Kyoto. All other chemicals and reagents used were of the highest grade available from commercial suppliers.
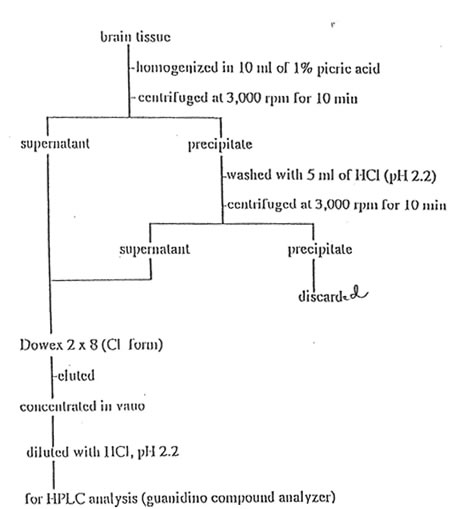
Analysis of Guanidino compounds The guanidino compounds in vacuoconcentrate prepared as shown in Fig. 1 was dissolved in 1.8 ml of HCI solution, pH 2.2 and analyzed by high pressure liquid chromatopraphy system JASCO G-250 guanidino compound analyzer (Tokyo) with electrochemical detector JASCO FP-100B fluorometer (Tokyo) using the PO reaction21,22. The analytical conditions employed were as column, Guanidino pak lit (6 x 35 mm, JASCO, Tokyo); temperature, 70oC; flowrate, 1.0 ml/min (eluent); flowrate, 0.5 ml/min (0.025% PQ in DMF); flowrate, 0.5 ml/min (0.1 M NaOH); reaction coil, 0.5 mm I.D. x 5 m L.; detector, fluorometer (JASCO FP-110, Ex 365 nm, Em 495 nm), and sample volume of 200 μl. Four eluents of 0.4 M sodium citrate buffer with different pH values and elution time corresponding to pH 3.0 at 10.2 min, pH 3.5 at 4.0 min, pH 5.25 at 4.2 min, and pH 10.0 at 9.4 min were used. The fifth eluent of 1 N NaOH was eluted within 5.2 min. The fluorescence reagent consists of 2 N NaOH and 0.025% PQ in DMF. Details are given in previous studies21.22.
Statistical analysis Statistical analysis was performed using Student’s t-test.
RESULTS
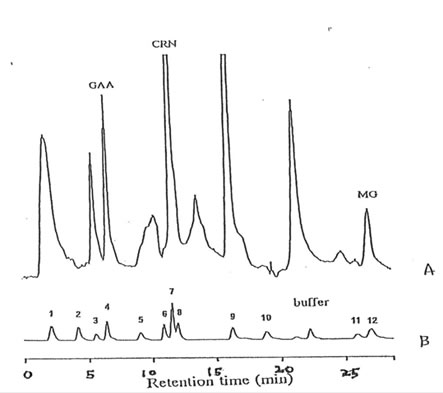
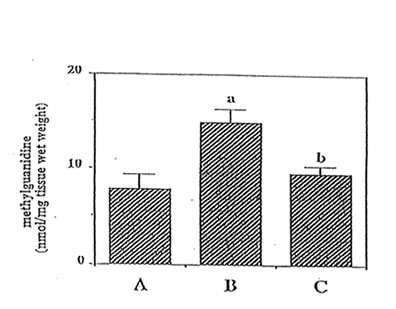
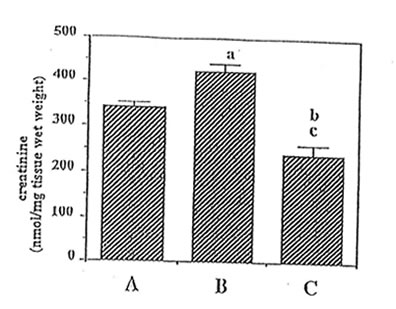
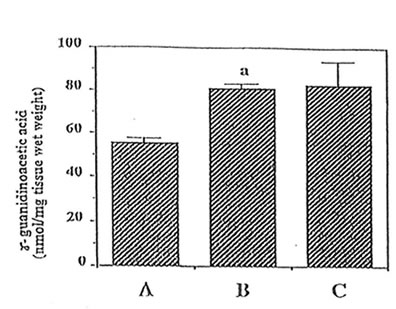
Important guanidino compounds related to the synthesis of MG such as CRN and GAA including MG were processed (as shown in Fig. 1), identified in the brain cortex of adult rats, aged rats and Bio-catalyzer-treated aged rats. Figure 2 shows the typical HPLC chromatogram of different guanidino compounds in the brain of these three groups of animals and the authentic sample of guanidino standards. The chromatograms reveal 5 peaks corresponding to GAA, CRN, Arg, HArg, and MG which were eluted at about 2.0, 11.5, 16.5, 20.0, and 26 min, respectively. For this study however, only GAA, CRN and MG were quantified.(9ompared to the adult brain, MG ( p < 0.025), CRN ( p < 0.025) and GAA ( p< 0.001) were increased in the aged rat brain (Fig. 3,4 and 5). The elevated levels of CRN (p < 0.005) and MG (p < 0.025) but not GAA in the brain of aged rats however, were suppressed by the 27-week oral administration of 0.1 g/kg BW of Bio-catalyzer.
DISCUSSION
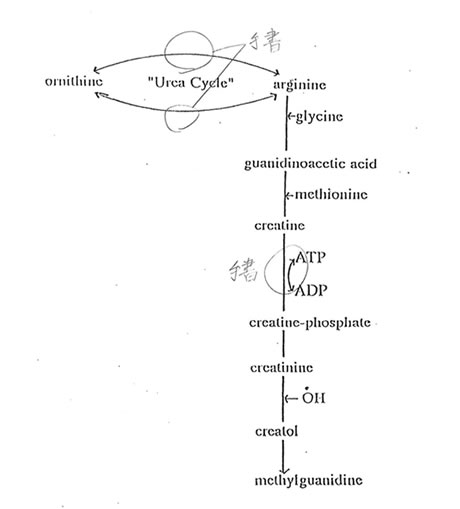
Reactive oxygen species (ROS) such as hydroxyl radicals with their ability to react with MG17,18 have been used as markers in the serum and plasma of hemodialyzed patients14 and in rat hepatocytes15,16. The convulsive activity and epileptic seizures in cats and rabbits19 and mice20 and spike discharges in the rat encephalograms26 were attributed to increased level in MG. The synthesis of MG has been suggested to be mediated by ROS. ROS, in particular hydroxyl radicals, may be generated from free hemoglobin which acts as biological Fenton reagent12 or iron promoter of Fenton reaction13. The neurotoxicology of MG has been reported27 and has been implicated in brain dysfunctions, such as epilepsy28,29 but its effect on ageing has not been studied. Most recently, we found that while Bio-catalyzer increased the elevated levels of superoxide dismutase; it inhibited the elevated levels of thiobarbituric acid-reactive substances (TBARS) in aged rat brain (unpublished data). Augmenting these observations, our findings reveal that MG and its precursors CRN and GM were significantly increased in the brain of aged rats. Figure 6 shows the possible metabolic pathway for the synthesis of methylguanidine in the brain. MG, which is hydroxyl radical-mediated and is a peroxidative product of CRN12,13 and creatol30, increases with a increase in CRN – indicative of the active participation of hydroxyl radical during the ageing process31. CRN was synthesized from GAA, hence an increased GAA led to an increase in CRN. These age-related increases in MG and CRN but not in GAA were suppressed significantly by Bio-catlyzer at 0.1 g/kg body weight of the animals – a dose that approximates the recommended dose for humans. Bio-catalyzer, a proven scavenger of hydroxyl radicals and an inhibitor of TBARS in iron-induced lipid peroxidative damage in epileptic foci in rats23, is thought to have inhibited the hydroxyl radical-mediated peroxidative conversion of CRN to MG, thus decreasing the levels of MG and CRN in aged rat brain. The results also indicate that the synthesis of CRN from GAA does not involve hydroxyl radical intermediates.
The current findings add to the evidence that a precursor CRN to MG synthesis is mediated by hydroxyl radical. MG may therefore be used as a marker radical formation in the brain and could prove useful in studying the mechanism of ageing and other brain dysfunctions believed to be caused by free radicals. The proposed metabolic pathway of CRN and MG in the brain may be similarly linked to that in liver and kidney in cases of acute and chronic renal failures 10.11.14-16,32. We speculate that Bio-catalyzer, a good inhibitor of hydroxyl radicals, may be an effective agent in arresting elevated levels of toxic convulsant MG in the brain of aged rats – indicative of its anti-convulsive and or anti-ageing properties. As an inhibitor of methylguanidine, Bio-catalyzer may also be a useful clinical agent in the amelioration of epilepsy, acute and chronic renal failure, among others.
REFERENCES
- Giovannetti, S.; Balestri. P.L.; Barsotti, G. Methylguanidine in uremia. Arch.Intern.Med. 131: 709-713; 1973.
- Cohen, B.D. Guanidino compounds: Implications in uremia. In: Mori, A.; Cohen, B.D.; Lowenthal, A.C., eds. Guanidines. Historical, biological, and clinical aspects of the naturally occurring guanidino compounds. New York: Plenum Press; 1985: 265-275.
- Koide, H.; Azushima, C. Metabolic profiles of guanidino compounds in various tissues of uremic hearts. In: Mori, A.; Cohen B.D.; Lowenthal A.C. eds. Guanidines. Historical, biological, and clinical aspects of the naturally occurring guanidino compounds. New York: Plenum press; 1985: 365-372.
- Yokoi, I.; Toma, J.; Mori, A. Effect of chronic alcohol administration on the concentrations of guanidino compounds in rat organs. In: Mori, A.; Cohen, B.D.; Lowenthal, A.C.; eds. Guanidines. Historical, biological, and clinical aspects of the naturally occurring guanidino compounds. New York: Plenum press; 1985: 249- 261.
- Hiramatsu, M. Guanidino compounds in mouse brain. I. Brain guanidino compound levels in twelve strains of mice. Okayama Igakkai Zasshi 92: 419; 1980:
- Mori, A.; Ichimura, T.; Matsumoto, H. Gas chromatography-mass spectrometry of guanidino compounds in brain. Anal Biochem. 89:393-399; 1978
- Matsumoto, M.; Kobayashi, K.; Mori, A. Distribution of guanidino compounds in bovine serum brain. J. Neurochem. 32: 645-646; 1979.
- Yokoi, I. Edaki, A.; Watanabe, Y.; Shiraga, H.; Fuji, T.; Mori, A. Guanidino compounds in rabbit brain, serum and other organs. Neurosciences 12: 27-32; 1986.
- Perez, G., Faluotico, R. Creatinine: A precursor of methylguanidine. Experientia 29: 1473-1474; 1973.
- Nagase, S.; Aoyagi, K, Narita, M.; Tojo, S. Biosynthesis of methylguanidine in isolated rat hepatocytes and in vivo. Nephron 40: 470-475; 1985.
- Nagase, S.; Aoyagi, K.; Narita, M.; Tojo, S. Active oxygen in methylguanidine synthesis. Nephron 44: 299-303; 1986.
- Sadrzadeh, S.M.; Graf, E.; Panter, S.S.; Hallaway, P. E.; Eaton, J.W. Hemoglobin – a biologic Fenton reagent. J. Biol. Chern. 259: 14354-14356; 1984.
- Gutteridge, J.M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett.-201: 29_t-295; 1986.
- Nakajima, M.; Nakamura, K.; Shirokane, Y.; Hirasawa, Y. Enzymic determination of methylguanidine in serum and plasma of hemodialysis patients as a marker for hydroxyl radicals. In: Mori, A.; Cohen, B.D.; Koide, H.; eds. Guanidines. 2. Further explorations of the biological and clinical significance of guanidino compounds. New York: Plenum Press; 1989: 3-11.
- Aoyagi, K.; Nagase, S.; Sakamoto, M.; Narita, M.; Tojo, S. Active oxygen in methylguanidine synthesis by isolated rat hepatocytes. In Mori, A.; Cohen, B.D.; Koide, H.; eds. Guanidines. 2. Further explorations of the biological and clinical significance of guanidine compounds. New York: Plenum Press; 1989:79-85.
- Aoyagi, K.; Nagase, S.; Sakamoto, M.; Narita, M.; Tojo, S. Puromycin aminonucleoside stimulates the synthesis of methylguanidine: A possible marker of active oxygen generation in isolated rat hepatocytes. In: Mori, A.; Cohen, B.D.; Koide, H.; eds Guanidines. 2. Further explorations of the biological and clinical significance of guanidine compounds. New York: Plenum Press; 1989: 71-77.
- Sakamoto, M.; Aoyagi K.; Nagase, S.; Ohba, S.; Miyazaki, M.; Narita, M.; Toji, S. Effect of. Active oxygen. On guanidine synthesis in vitro In: Mori, A.; Cohen, B.D.; Koide, H.; eds. Guanidines. 2. Further explorations of the biological and clinical significance of guanidine compounds. New York: Plenum Press; 1989: 87-95.
- Hiramatsu, M.; Edamatsu, R.; Kohno, M.; Mori, A. The reactivity of guanidino compounds with hydroxyl radicals. In: Mori, A.; Cohen, B.D.; Koide, H.; eds. Guanidines. 2. Further explorations of the biological and clinical significance of guanidino compounds. New York: Plenum Press; 1989:97-104.
- Matsumoto, M.; Kobayashi, K.; Kishikawa, H.; Mori, A. Convulsive activity of methylguanidine in cats and rabbits. IRCS Med Sci. 4: 65; 1976.
- Hiramatsu, .M. Guanidino compounds in mouse brain. II. Guanidino compound levels in brain in relation to convulsions. Okayama Igakkai Zasshi.92: 427; 1980.
- Mori, A.; Katayama, Y.; Higashidate, S.; Kimura, S. Fluorometrical analysis of guanidine compounds in mouse brain. J. Neurochem. 32: 643-649; 1979.
- Mori, A.; Watanabe; Y.; Fujimoto, N. Fluorometrical analysis of guanidine compounds in human cerebrospinal fluid. J Neurochem. 38: 448-450; 1982.
- Santiago, L.A.; Osato, J.A.; Hiramatsu, M.; Edamatsu, R.; Mori, A. Free radical scavenging action of Bio-catalyzer α·ρ No. 11 (Bio normalizer) and its by-product. Free Radical Biol Med.11: 379-363; 1991.
- Glowinski, J.; Iversen, L. Regional studies of catecholamine in the rat brain. J. Neurochem. 13: 655-669; 1966.
- Mori, A.; Akagi, M.;Katayama, Y.; Watanabe, Y- Guanidinoglutaric acid in cobalt induced epileptogenic cerebral cortex of cats. J. Neurochem.35: 603-605; 1980.
- Yokoi, I.; Shimizu, Y.; Ohba, S.; Mori, A Effects of GABAergic drugs on spike discharges induced by methylguanidine in the rat electroencephalograms. Neurosciences 16: 359-364; 1990.
- Mori, A. Biochemistry and neurotoxicology of guanidino compounds: History and recent advances. Pavlov.J.Biol.Sci. 22:85-94; 1987.
- Plum, C.M. Studies on the occurrence of guanidino compounds in serum and urine of patients with epilepsy, neuronal ceroid-lipofuscinosis and uremic patients. In: Mori, A.; Cohen, B.D.; Lowenthal, A. eds; Guanidines. Historical, biologica,l biochemical, and clinical aspects of the naturally occurring guanidino compounds. New York: Plenum Press; 1985: 159-169.
- Shiraga, H.; Watanabe, Y.; Mori, A. Guanidino compound levels in the serum of healthy adults and epileptic patients. Epilepsy Res.8: 142-148; 1991.
- Nakamura, K.; lenaga, K.; Yokozawa, T.; Fujitsuka, N.; Oura, H. Production of methylguanidine from creatinine via creatol by active oxygen species: Analyses of the catabolism in vitro.
- Sawada, M.; Carlson, J.C. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech. Aging. Dev. 41: 125-137; 1987.
- Gejyo, F.; Baba, S.; Watanabe, Y.; Kishore, B.K.; Suzuki, Y.; Masaaki, A. Possibility of a common metabolic pathway for the production of methylguanidine and dimethylamine in chronic renal failure. In: Mori, A.; Cohen, B.D.; Lowenthal, A. eds.; Guanidines. Historical, biological, biochemical, and clinical aspects of the naturally occurring guanidino compounds. New York: Plenum Press; 1985: 295-308.
FIGURE LEGEND
Fig. 1. Isolation of guanidino compounds in rat brain tissue.
Fig. 2. HPLC chromatogram of guanidino compounds in the cortex of rats orally administered with 0.1 g/kg body weight of Bio-catalyzer for 27 consecutive weeks (A), and of the standard sample. (1) guanidinoethane sulfonate, GES; (2) guanidinosuccinic acid, GSA; (3) guanidinoglutaric acid, GGA; (4) guanidinoacetic acid, GAA; (5) N-acetylarginine, NM; (6) guanidinopropionic acid, GPA; (7) creatinine, CRN; (8) guanidinobutyric acid GBA; (9) arginine, ARG; (10) homoarginine, HAAG; (11) guanidine, G; and (12) methylguanidine, MG.
Fig. 3. Effect of oral administration of 0.1 g/kg body weight of Bio-catalyzer on elevated level of methylguanidine in aged rat brain. (A) adult rat, (B) aged rat, and (C) treated-aged rat. Values are means+ SEM of 5-6 animals. ap < 0.025 versus (A); bp < 0.025 versus (8).
Fig. 4. Effect of oral administration of 0.1 g/kg body weight of Bio-catalyzer on elevated level of creatinine in aged rat brain. (A) adult rat, (B) aged rat, and (C) treated aged rat.Values are means + SEM of 4 animals. a.bp < 0.025 versus (A);cp < 0.005 versus (B).
Fig. 5. Effect of oral administration of 0.1 g/kg body weight of Bio-catalyzer on elevated
level of – guanidinoacetic acid in aged rat brain. (A) adult rat, (B) aged rat, and (C) treated-aged rat. Values are means+ SEM of 6 animals .ap < 0.001 versus (A).
Fig. 6. Metabolic pathway of methylguanidine synthesis in the brain.
Figure1
Santiago et al
Inhibitory action of Bio-catalyzer on methylguanidine
Figure 2
Santiago et al
Inhibitory action of Bio-catalyzer on methylguanidine
Figure 3
Santiago et al
Inhibitory action of Bio-catalyzer on methylguanidine
Figure 4
Santiago et al
Inhibitory action of Bio-catalyzer on methylguanidine
Figure 5
Santiago et al
Inhibitory action of Bio-catalyzer on methylguanidine
Figure6
Santiago et al
Inhibitory action of Bio-catalyzer on methylguanidine