| Title | AGE-RELATED INCREASES IN SUPEROXIDE DISMUTASE ACTIVITY AND THIOBARBITURIC ACID-REACTIVE SUBSTANCES: EFFECT OF BIO-CATALYZER IN AGED RAT BRAIN |
|---|---|
| Year | 1993 |
| Author | Librado A Santiago, James Akira Osato, Jiankang Liu, and Akitane Mori |
| Publisher | Neurochemical Research |
Age-Related Increases in Superoxide Dismutase Activity and Thiobarbituric Acid-Reactive Substances: Effect of Bio-catalyzer in Aged Rat Brain
Librado A Santiago,1 James Akira Osato,2 Jiankang Liu,1 and Akitane Mori1,3
(Accepted October 29, 1992)
Abstract: This study describes, using electron spin resonance spectrometry/spin trapping technique, the increase superoxide dismutase (SOD) activity in the mitochondrial and cytosolic fraction of the cortex, midbrain, pons-medulla oblongata and cerebellum, and in thiobarbituric acid-reactive substances (TBARS) in the cortex, cerebellum and hippocampus of the aged rats. The results show that corresponding to the increased life span and improved physical conditions observed after peroral long-term treatment with Bio-catalyzer, a commercial natural fermented health food supplement marketed in Japan and in the Philippines and earlier reported to be a hydroxyl radical scavenger with weaker scavenging activity on superoxide radical (O–2), SOD which is involved in the metabolic degradation of O–2 was further increased, whereas TBARS decreased. These findings suggest that the increased SOD activity in the brain as a defense mechanism against age-related accumulation of reactive oxygen species, in particular superoxide radicals, was enhanced with Bio-catalyzer treatment while age-related peroxidation of neuronal membrane, as measured by TBARS, was decreased.
Keywords: Superoxide dismutase; thiobarbituric acid-reactive substances; aging; lipid peroxidation; free radical inhibitor; antioxidant; Bio-catalyzer.
Introduction
The free radical theory postulates that free radical reactions contribute to the aging process (1-6). Reactive oxygen species (ROS) are generated naturally or introduced in living cells affecting most of the biological molecules containing polyunsaturated fatty acids (PUFA), DNA and proteins. However, cells are endowed with an array of antioxidants such as vitamins A, C, and E, glutathione, among others, and also by different protective enzymes. One of these is superoxide dismutase (SOD), an abundant enzyme found in the mitochndria (Mn-SOD) and in the cytosol (CuZn-SOD), which catalyzes the dismutation of superoxide radical (O–2) into H2O2. Divergent reports on SOD show that it decreases (7-9), increases (10-13) or remains unchanged (14-22) in the brain of aged rodents. In this study, we used electron spin resonance (ESR) spectrometry/spin trapping technique, the least ambiguous method for determining free radicals (23), to measure the activity of Mn-SOD and CuZn-SOD in the different brain regions of aged rats.
1 Department of Neuroscience, Institute of Molecular and Cellular Medicine, Okayama University Medical School, 2-5-1 Shikata-cho, Okayama
700, Japan
2 Sun-O International Inc., Gifu 500, Japan
3 Address reprint to: Akitane Mori, M.D., Ph.D., Chairman,Department of Neuroscience, Institute of Molecular and Cellular Medicine, Okayama
University Medical School, 2-5-1 Shikata-cho, Okayama 700, Japan
The damaging effects of ROS and the rapid accumulation of peroxidative products such as thiobarbituric acid-reactive substances (TBARS), are important mechanisms implicated in many pathological diseases and disorders. We analyzed the level of TBARS in the brain of aged rats – the organ most sensitive to ROS damage because of its high level of PUFA and high oxygen consumption rate.
Life expectancy in Japan in the past decade has dramatically increased with the Japanese having the world’s highest longevity since 1987 (1). The reason behind such increase is not altogether clear, but it certainly includes better nutrition and health care.
Increasing the intake of free radical inhibitors has been suggested to blunt the loss of key cytosolic enzymes and antioxidants and to protect the neuronal membrane from peroxidation (1,24,25). The clinical use of antioxidant or free radical inibitor as a new therapeutic strategies for the prevention and attenuation of aging has thus generated much interest.
Bio-catalyzer (BC), a natural fermented health food supplement from Carica papaya Linn. and other tropical herbal plants with fermentative cereals from Japanese tropical and traditional foods (26), and commercially available in Japanese and Philippine markets, has been observed to exhibit diverse and wide-ranging biological, physiological and clinical actions. The hydroxyl radical scavenging property of BC (26,27) and its ability to inhibit TBARS in rats intracortically injected with FeCl3 (26) (an experimental model for post-traumatic epilepsy) paved the way for further studies.
To elucidate the antioxidant defense mechanism(s) of BC on aging, we examined its effect on SOD activities and TBARS levels in the different regions of the brain of aged rats by ESR spectrometry/spin trapping technique and fluorometry.
Experimental Procedure
Bio-catalyzer. Bio-catalyzer a.r No.11 (Bio-normalizer) is a white, sweet health food preparation produced by fermentation of Carica papaya Linn. (a Philippine medicinal plant) and other tropical herbal plants (26) and is available commercially from Sun-O International Inc., Gifu, Japan.
Chemicals. Hypoxanthine, diethylenetriaminepentaacetic acid (DETAPAC) and SOD (2,800 units mg-1 protein) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Xanthine oxidase (XOD, cow’s milk) and 5,5’-dimethyl-1-pyrroline-N-oxide (DMPO) were purchased from Boehringer GmbH (Mannheim, Germany) and Daiichi Pure Chemicals Co., Ltd. (Tokyo, Japan), respectively. All other chemicals and reagents were of the highest grade available from commercial suppliers.
Animals. Male Sprague-Dawley rats from Clea Japan Inc., (Tokyo, Japan) aged 12 and 89 weeks were purchased and experimented for 27 weeks. Thirty five aged rats (89 week-old) were housed in individual aluminum cages at 20-26oC, humidity of 40-70%, ventilation of 15-25 times/h, light of 150-160 lux for 12h/day from 7:00 to 19:00, noise <60 phon, ammonia of <20 ppm, air stream of 25 cm/sec and were maintained under strict sanitary conditions. The animals were allowed free access to water and a standard diet (CE-2 pellet supplied by Clea Japan, Inc., Tokyo). The rats were divided randomly into two groups; the control group consisting of 20 rats orally administered with tap water boiled for 30 min while the second group of 15 rats was orally administered with 0.1 g/kg body weight (BW) of BC for 27 consecutive weeks (189 days) at a volume dosage of 1.0 ml/100 g animal weight. This dose approximates the recommended dose for humans which is 3-9 g/day. The administration was done from 14:00 to 17:00 after 2-3 h period of restricted food and water. The rats were sacrificed on the 27th week by cervical decapitation. The cortex, midbrain, pons-medulla oblongata, cerebellum, hippocampus and striatum were dissected on an ice plate (28), frozen in liquid nitrogen and kept at –80oC until analysis.
Measurement of SOD Activity. Analysis of SOD activity (10) was performed by electron spin resonance (ESR) spectrometry using spin trapping technique. The brain tissues were homogenized in 10 volumes of 0.1 M sodium phosphate buffer (pH 7.4) and centrifuged at 1650 g for 15 min. The supernatant was re-centrifuged at 11,000 g for 30 min and the crude mitochondrial pellet obtained was suspended in the same buffer. This suspension was used as the mitochondrial fraction. The resulting supernatant obtained after the second centrifugation was then ultracentrifuged at 105,000 g for 60 min and the supernatant was used as the cytosolic fraction. The SOD activity was measured by ESR spectrometer (JEOL, JES-FE1XG, Tokyo, Japan) using spin trapping agent DMPO. Fifty microliters of 2 mmol/L hypoxanthine, 35 ml of 5.5 mmol/L DETAPAC, 50 ml of sample, 15 ml of DMPO and 50 ml of XOD (0.326 unit/ml) were mixed in a test tube by automatic mixer. The solution was placed in a 169 ml-volume capacity special flat cell (JEOL, Tokyo, Japan) and the superoxide spin adduct (DMPO-OOH) was recorded exactly after 1 min of mixing. The conditions for ESR analysis were as follows: magnetic field 335 ± 5 mT; power, 8 mW; response, 0.1s; modulation, 0.2 mT; temperature, room temperature; amplitude, 1.25 x 103; and sweep time, 2 min. A standard SOD curve was prepared from different diluted concentrations of SOD in 0.1 M sodium phosphate buffer (pH 7.4) using manganese oxide as an internal standard. The spin number was calculated using the ratio signal height intensity of 2,2,6,6-tetramethyl-4-hydroxylpiperidine-1-oxyl standard of known spin quantity. The SOD activity was expressed as unit/mg protein.
Protein Assay. The protein concentration was determined by Pierce BCA Protein Assay Kit (Rockford, Illinois 61105 USA) with bovine serum albumin as the standard and the absorbance was read at 562 nm.
Measurement of TBARS Formation. Evaluation of TBARS (29) was performed on 0.1 ml of brain homogenate (10% in 0.1 M sodium phosphate buffer pH 7.4) by adding a) 0.2 ml of 8.1% sodium dodecyl sulfate, b) 1.5 ml of 20% of sodium acetate solution (adjusted to pH 3.5 with 0.1 N NaOH, c) 1.5 ml of 0.8% aqueous sodium thiobarbituric acid (TBA), and d) 0.7ml of distilled water; then mixed and incubated at 100°C in a water bath for 60 min. After cooling, 1 ml of distilled water and 5 ml of the n-butanol and pyridine mixture (15:1 v/v) were added and shaken vigorously for 1 min. After centrifugation at 3000 rpm for 10 min, the absorbance of the supernatant containing the TBA-reactive substances (TBARS) was read at Ex 515 nm and Em 532 nm using a fluorometer. The values were determined by a standard curve of 1,1,3,3-tetramethoxypropane. The formed lipid peroxide was expressed as nmol TBARS/g wet weight tissue.
Statistical analysis. Statistical analysis was performed by Chi-square test and Student’s t-test wherever applicable.
Results
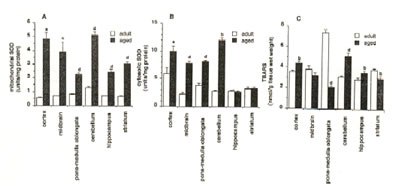
Compared to the adult rat brain tissue homogenates, the mitochondrial SOD activity levels in the aged rat brain significantly increased in the cortex (p<0.005), midbrain (p<0.01) and pons-medulla oblongata, cerebellum, hippocampus and striatum (p<0.001) (Figure 1A).
The cytosolic SOD levels showed remarkable increases in the cortex (p<0.05) and in the midbrain, pons-medulla oblongata (p<0.001) and cerebellum(p<0.025) of the aged rats and no changes in the hippocampus and striatum (Figure 1B).
In the TBARS analysis, the aged rat brain showed increased level in the cortex (p<0.025), cerebellum (p<0.001) and hippocampus (p<0.025). The levels however, significantly decreased in the pons-medulla oblongata (p<0.001) and striatum (p<0.025) while no change was observed in the midbrain (Fig. 1C).
The Bio-catalyzer-treated aging rodents generally showed improved physical conditions than the untreated group (control) with the former having more lustrous and healthy coats, were bright eyed and agile, and showed none of the immobility problems associated with old age in rats.
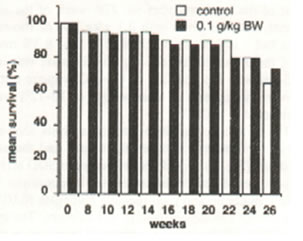
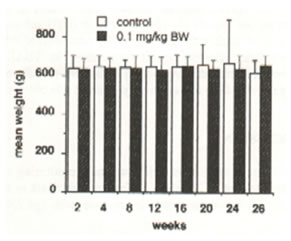
Figure 2 shows no significant change on the mean percentage survival rate of aging rats for a 26 week-period of Bio-catalyzer treatment. Figure 3 indicates no remarkable difference on the mean weight between the control and treated aged rats for a 26-week period (Figure 3). Table 1 shows the mean survival rate and mean weight of the aged rats after the 27th week of the experiment. The rats given with 0.1 g/kg BW of Bio-catalyzer had a higher survival rate of 73.3%, 8.3% over that of the untreated rats although the increase was insignificant with a probability of 0.44 (Chi-square test). However, the increase in mean weight of 10-12% was significant at p<0.025.
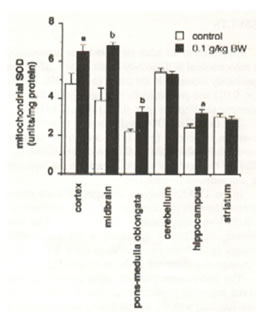
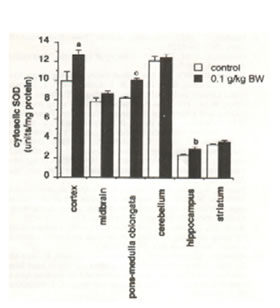
The mitochondrial SOD activity analysis showed remarkable disparities between the treated and the untreated rats (Fig. 4). Significant increases in SOD activity levels were found in the cortex and hippocampus (p<0.05), midbrain and pons-medulla oblongata (p<0.025) but no changes in the cerebellum and striatum. The cytosolic SOD activity level significantly increased in the cortex (p<0.05), pons-medulla oblongata (p<0.001) and hippocampus (p<0.025). No difference was found in the midbrain, cerebellum and striatum (Figure 5).
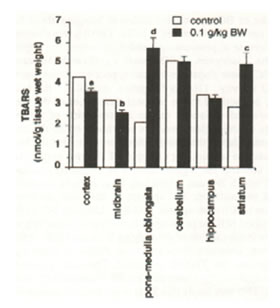
The TBARS analysis showed varied results in the brain regions (Figure 6). The cortex (p<0.05) and midbrain (p<0.025) of aged rats orally administered with Bio-catalyzer showed significant decrease in TBARS. However, increased TBARS level in the pons-medulla oblongata (p<0.005) and striatum (p<0.01) was observed.
Fig.1. Age-related changes on A) Mitochondrial superoxide dismutase (SOD) activity, B) cytosolic SOD activity and C) thiobarbituric acid-reactive substances (TBARS) in aged rat brain. Values are means ± SEM of 6-13 animals p<0.05 (a), p<0.025 (b),p<0.01 (c), and p<0.001 (d) versus control.
Fig.2. Effect of oral administration of 0.1 g/kg body weight of Bio-catalyzer for 26 weeks on the mean percentage survival of aged rats. Values are means of 11-20 animals.
Fig.3. Effect of oral administration of 0.1 g/kg body weight of Bio-catalyzer for 26 weeks on the mean weights of aged rats. Values are means of 11-20 animals.
Fig.4. Effect of oral administration of 0.1 g/kg body weight of Bio-catalyzer for 27 weeks on mitochondrial superoxide dismutase activity in aged rat brain. Values are means ± SEM of 6-13 animals. p<0.05(a), p<0.025 (b), and p<0.001 (c) versus control.
Table I. Effect of Bio-catalyzer on the Mean Percentage Survival and Mean Weight of Aged Rats After 27 weeks of Oral Administration
Values are means ± SEM of 11 animals. Increase not significant at ap = 0.44 (Chi-square test); bp<0.025.
Fig.5. Effect of oral administration of 0.1 g/kg body weight of Bio-catalyzer for 27 weeks on cytosolic superoxide dismutase activity in aged rat brain. Values are means ± SEM of 6-10 animals.p<0.05 (a), p<0.025 (b), and p<0.001 (c) versus control.
Fig.6. Effect of oral administration of 0.1 g/kg body weight of Bio-catalyzer for 27 weeks on thiobabituric acid-reactive substances (TBARS) in aged rat brain. Values are means ± SEM of 10 –13 animals. p<0.05 (a), p<0.025 (b), p<0.01 (c), and p<0.005 (d) versus control.
Discussion
There is now substantial evidence implicating the involvement of O–2 in tissue injury and cell death in the brain. O–2 has been shown to increase with age (30). About 1% of the oxygen used in the mitochondria is transformed to O–2, with a daily output as high 107 as molecules of O–2/mitochondrion/day (31). Most mitochondrial O–2 is destroyed by Mn-SOD whereas cytosolic O–2 is dismutated by CuZn-SOD.
Mn-SOD activity in the brain of aged rats as measured by ESR/spin trapping technique was found to increase with age in all parts studied. Similarly, age-dependent increases in CuZn-SOD in all brain regions, except in the hippocampus and striatum (which were unchanged) were observed. This occurrence coincides with age-dependent increases in the formation of O–2 (30). The data in general are in agreement with those of other studies (11-13) including the previous ESR investigation on aged rat brain (10).
However, the detoxification of SOD on O–2 is not a terminating step since, as a result of the enzyme-catalyzed dismutation, H2O–2 is produced and accumulates in the mitochondria or cytosol. In turn, H2O2 which may not be totally inhibited by catalase and glutathione peroxidase (more potent antioxidant enzymes than SOD), reacts with O–2 to form an extremely reactive hydroxyl radical (.OH). Monoamine oxidase, xanthine oxidase, glycine oxidase, NADH dehydrogenase, NADPH oxidase, among others, generate H2O2. H2O2 in the presence of iron may also lead to the production of .OH (32). These oxygen species which may attack DNA (33,34), proteins (35) and lipids (14), in addition to singlet molecular oxygen, are believed to cause lipid peroxidation chain reactions and widespread cellular injury in the aging process.
Catalase and glutathione peroxidase have been found to decrease with age (11,36). It is estimated that 25% of enzyme activities are lost during adulthood senescence (36). This deficiency may indicate a lowered resistance with age, and when production of H2O2 and .OH, singlet oxygen, and peroxides are excessive, tissue damage as measured by increased TBARS, can occur. Our findings show increased levels of TBARS in cortex, cerebellum and hippocampus; decreased levels in pons-medulla oblongata and striatum; and no change in midbrain during aging. The results are similar to those obtained by other investigators (21,30). The varied results may be ascribed to the fact that the generated free radicals may or may not be eliminated efficiently, due to perhaps variations in antioxidants and antioxidant enzymes or perturbed enzyme activities in the different brain regions during aging.
Thus, the study of systems for additional protection against free radicals becomes imperative, both in neurotoxicology and in the search for new therapeutic strategies on disease prevention and slowing down aging. BC, a natural product with purported clinical uses in humans, was unequivocably demonstrated by ESR/spin trapping technique to scavenge hydroxyl radicals and to a weaker degree, O–2 in vitro; and to inhibit TBARS formation in iron-induced epileptic focus in rats (26). BC also inhibited the excitatory release of neuromediators dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, serotonin, and 5-hydroxyindoleacetic acid in the striatal perfusate of the rat intracortically injected with FeCl3 (unpublished data). The hydroxyl radical scavenging components of BC, which are thought to be responsible for these phenomena, appear to be multiple, thermostable and acid-tolerant (27). However, the specificity at molecular level of the active components need further characterization.
The peroral long-term administration of BC on aging rats improved their physical conditions, increased remarkably their weight and prolonged their life span by 8.3% as compared to the control group. The weight gain is likely derived from the additional calories (3.66 kcal/g) or other unknown health benefits of BC. BC (0.1 g/kg BW) was compulsory administered daily at a volume dosage of 1.0 ml/100 g BW after 2-3 h period restricted food and water. BC manifested a protective action with further increase in Mn-SOD in the cortex, hippocampus, midbrain and pons-medulla oblongata and in CuZn-SOD in the cortex, pons medulla oblongata and hippocampus. TBARS level decreased in the cortex and midbrain but increased in the striatum and pons-medulla oblongata inspite of BC’s protective action in bringing about further age-related increases in SOD. TBARS was observed to increase in pons-medulla oblongata where the activity of the 2 isozymes of SOD were significantly inceased by BC. These disparities indicating poor correlation among SOD activity, TBARS formation and BC in the brain may be ascribed to the regional differences of the brain tissues and variations on the concentration of antioxidants or antioxidant enzymes which determine the level of antioxidative stress and radical formation that the brain tissues can sustain. For example, of all brain parts, striatum contains the highest amount of dopamine, antioxidant which BC can inhibit and thus, is more subject to lipid peroxidation. BC appears to have less inhibitory action on striatum against TBARS. Although BC further increased SOD in pons-medulla oblongata, since this enzyme is not capable of detoxifying H2O2 and .OH, which in turn, abstracts hydrogen from PUFA to form lipid peroxides, the TBARS increased. The same reasoning may apply to the observed TBARS increase in the striatum. This may imply that SOD is not the sole mechanism that operates against these radicals, rather it may be an important mechanism in aging that operates in conjunction with catalase, glutathione peroxidase, vitamin E and ubiquinone (intramembrane protectors), glutathione and vitamin C (reducing agents), selenium and dopamine in the brain. The complex interactions between the nutrients of BC and these cellular antioxidant defense systems can lead to imbalances in the oxidation-reduction status of the brain tissues with some of them being more susceptible to alterations than others. Hence, the effects of BC on the brain have to be seen as a result of multiple effects, a mixture of natural products in itself, as in most food and medicinal plant preparations (37-42), such that one oxidative component may increase the need for another and thus result in beneficial or undesirable effects. Some possible antioxidative components of BC are papaya (43-45), yeast (46) and glucose.
The increase of antioxidant enzyme SOD in most brain regions, and the inhibition of TBARS suggest that oxygen-derived radicals such as O–2, .OH, H2O2, and peroxides, may have some effects on premature aging. With its ability to suppress TBARS in most brain parts, BC may be considered as a rational approach in retarding aging and attenuating other age-related neurological dysfunctions caused by free radicals.
References
- Harman, D. 1991. The aging process: Major risk factor for disease and death. Proc. Natl. Acad. Sci. USA. 88:5360-5363.
- Harman, D. 1981. The aging process. Proc. Natl. Acad. Sci. USA. 78:7124-7128.
- Harman, D. 1984. Free radical theory o f aging: the “free radical” disease. Aging. 7:111-113.
- Harman, D. 1987. The free radical theory of aging. Pages 81-87 in H.R. Warner, R.N. Butler, R. L. Sprott, and E.L. Schneider (eds.) Modern biological theories of aging N.Y. Raven Press.
- Pryor, W. A. 1987. The free radical theory of aging revisited: A critique and a suggested disease specific theory. Pages 89-112. in H.R. Warner, R.N. Butler, R. L. Sprott, and E.L. Schneider (eds.) Modern biological theories of aging N.Y. Raven Press.
- Yoshikawa, T. Naito, Y., and Kondo, M. 1990. Free radical involvement in the aging process. Neurosciences. 16:603-612.
- Rao, G., Xia, E., and Richardson, A. 1990. Effect of age on the expression of antioxidant enzymes in male Fisher F344 rats. Mech. Aging Dev. 53:49-60.
- Vanella, A., Villa, R.F., Gorini, A., Campisi, A., and Giuffrida-Stella,A. M. 1989. Superoxide dismutase and cytochrome oxidase activities inlight and heavy synaptic mitochondria from rat cerebral cortex during aging. J. Neurosci. Res. 22:351-355.
- Benzi, G., Pastoris, O., and Villa, R. F. 1988. Changes induced by aging and drug treatment on cerebral enzymatic antioxidant system. Neurochem Res. 13:467-478.
- Hiramtsu, M., Kohno,M., Edamatsu,R., Mitsuta, K., and Mori, A. 1992. Increased superoxide dismutase activity in aged human cerebrospinal fluid and rat brain determined by electron spin resonance spectrometry using the spin trap method. J. Neurochem.58:1160-1164.
- Dahn, H. C., Benedetti, M. S., and Dostert, P. 1983. Differential changes in superoxide dismutase activity in brain and liver of old rats and mice. J. Neurochem. 40:1003-1007.
- Tolmasoff, J.M., Ono, T., and Cutler, R. G. 1980. Superoxide dismutase: Correlation with life span and specific metabolic rate in promate species. Proc. Natl. Acad. Sci. USA. 77:2777-2781.
- Nistico, G., Ciriolo, M.R., Fiskin, K., Iannone, M., de Martino, A., and Rotilio, G. 1992. NGF restores decrease in catalase activity and increases superoxide dismutase and glutathione peroxidase activity in the brain of aged rats. Free Radical Biol. Med. 12:177-181.
- Kellogg, E.W. III, and Fridovich, I. 1976. Superoxide dismutase in rat and mouse as a function of age and longevity. J. Gerontol. 31:405-408.
- Ansari, K.A., Kaplan, E., and Shoeman, D. 1989. Age-related changes in lipid peroxidation and protective enzymes in the central nervous system. Growth, Dev. And Aging. 53:117-121.
- Reiss, U., and Gershon, D. 1976. Comparison of cytoplasmic superoxide dismutase in liver, heart and brain of aging rats and mice. Biochem. Biophys. Res. Commun. 73:255-262.
- Cand, F., and Verdetti, J. 1989. Superoxide dismutase, glutathione peroxidase, catalase, and lipid peroxidation in the major organs of the aging rats. Free Radical Biol. Med. 7:59-63.
- de Quiroga, G.B., Perez-Campo, R., and Lopez, T.M. 1990. Antioxidant defenses and peroxidation in liver and brain of aged rats. Biochem. J. 272:247-250.
- Geremia, E., Baratta, S.D., Zafarana, S., Giordano, R., Pinizotto, M.R., La Rosa, M.G., and Garozzo, A. 1990. Antioxidant enzymatic systems in neuronal and glial cell-enriched fractions of rat brain during aging. Neurochem. Res. 15:719-723.
- Kurobe, N., Suzuki, F., Kato, K., and Sato, T. 1990. Sensitivity immunoassay of rat Cu/Z superoxide dismutase: concentrations in the brain, liver, and kidney are not affected by aging. Biomed. Res. 11:187-194.
- Mizuno, Y., and Ohta, K. 1986. Regional distributions of thiobarbituric acid-reactive products, activities of enzymes regulating the metabolism of oxygen free radicals, and some of the related enzymes in adult and aged rat brains. J. Neurochem. 46:1344-1352.
- Semsei, I., Rao, G., and Richardson, A. 1989. Changes in the expression of superoxide dismutase and catalase as a function of age and dietary restriction. Biochem. Biophys. Res. Commun. 164:620-625.
- Buettner, G.R. 1987. Spin trapping: ESR parameters of spin adducts. Free Radical Biol. Med. 3:259-303.
- Kim, J.W., and Yu, B. P. 1989. Characterization of age modulated malondialdehyde oxidation: the effect of modulation with food. Mech. Aging Dev. 50:277-287.
- Laganiere, S., and Yu, B.P. 1989. Effect of chronic food restriction in aging rats. II. Liver cytosolic antioxidants and related enzymes. Mech. Aging Dev. 48:221-230.
- Santiago, L.A., Osato, J.A., Hiramatsu, M., Edamatsu, R., and Mori, A. 1991 Free radical scavenging action of Bio-catalyzer a.r No.11 (Bio-normalizer) and its by-product. Free Radical Biol. Med. 11:379-383.
- Santiago, L.A., Osato, J.A., and Mori, A. 1992. Stability of the hydroxyl radical scavenging components of the health food “Bio-normalyzer”. Med. Sci. Res. 20:27-28.
- Glowinski, J., and Iversen, L. 1966. Regional studies of catechoamines in the rat brain. J. Neurochem. 13:655-669.
- Ohkawa, H., Ohishio, S., and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95:351-358.
- Sawada, M., and Carlson, J.C. 1 987. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech. Ageing Dev. 41:125-137.
- Richter, C. 1988. Do mitochondrial DNA fragments promote cancer and aging? FEBS Lett. 241;1-5.
- Burkitt, M.J., and Mason, R. P. 1991. Direct evidence for in vivo hydroxyl-radical generation in experimental iron overload: An ESR spin-trapping investigation. Proc. Natl. Acad. Sci. USA 88:8440-8444.
- Hart, R.W., and Setlow, R.B. 1974. Correlation between deoxyribonucleic acid and excission-repair and life span in a number of mammalian species. Proc. Natl. Acad. Sci. USA. 71:2169-2173.
- Adelman, R., Saul, R.L., and Ames, B. N. 1988. Oxidative damage to DNA: Relation of species metabolic rate and life span. Proc. Natl. Acad. Sci. USA. 85:2706-2708.
- Oliver, C.N., Ahn, B.W., Moerman, E.J., Goldstein, S., and Stadtman, E.R. 1978. Age-related changes in oxidized proteins. J. Biol. Chem. 262;5488-5491.
- Roth, G.S. 1979. Hormone receptor changes during adulthood senescence: significance for aging research. Fed. Proc. 1910-1914.
- Santiago, L.A., Hiramatsu, M., and Mori, A. 1992. Japanese soybean paste miso scavenges free radicals and inhibits lipid peroxidation. J. Nutr. Sci. Vitaminol. 38:297-304.
- Hiramatsu, M., Haba, K., Edamatsu, R., Hamada, H., and Mori, A. 1989. Increased choline acetyltransferase activity by Chinese Herbal Medicine Sho-saiko-to-go-keishi-ka-shakuyaku-to in aged rat brain. Neurochem. Res. 14:249-251.
- Haba, K., Ogawa, N. and Mori, A. 1990. The effects of sho-saiko-to-go-keishi-ka-shakuyaku-to (TJ-960) on ischemia-induced changes of brain acetycholine and monoamine levels in gerbils. Neurochem. Res. 15:487-493.
- Mori, A., Hiramatsu, M., Hamada, H., and Edamatsu, R. 1990. Aging and herbal medicine – Neuropharmacology of TJ-960. Neurosciences 16:83-88.
- Kabuto, H., Yokoi, I., and Mori, A. 1992. Monoamine metabolites, iron-induced seizures, and the anticonvulsant effects of tannins, Neurochem. Res. 17:585-590.
- Liu, J., Edamatsu, R., Kabuto, J., and Mori, A. 1990. Antioxidant action of Guilingji in the brain of rats with FeCl3-induced epilepsy. Free Radical Biol. Med. 9:451-454.
- Webman, E.J., Edlin, G., and Mower, H.F. 1989. Free radical scavenging action of papaya juice. Int. J. Radiat. Biol. 55:347-351.
- Gray, J. and Mower, H.F. 1991. The role of simple carbohydrates in the suppression of hydroxyl free radicals in g-irradiated papaya juice. Food Chem. 41:293-301.
- Santiago, L.A., Osato, J.A., and Mori, A. 1992. Free radical scavenging action of Carica papaya Linn. Neurosciences 18:189-194.
- Santiago, L.A., Hiramatsu, M., and Mori, A. 1991. Scavenging action on free radicals and inhibition of lipid peroxidation by baker’s yeast. Med. Sci. Res. 19:867-868.