| Title | EFFECTS OF BIONORMALIZER ALONE OR IN COMBINATION WITH ANTICANCER DRUGS ON CANCER CELLS. A PRELIMINARY STUDY |
|---|---|
| Year | |
| Author | R. Ortega; M. Simonoff; J.A. Osato; L.A. Santiago |
| Publisher | Centre de’Etudes Nucleaires de Bordeaux Gradignan, France |
EFFECTS OF BIONORMALIZER ALONE OR IN COMBINATION WITH ANTICANCER DRUGS ON CANCER CELLS.
A PRELIMINARY STUDY
ORTEGA Richard, SIMONOFF Monique
Centre de’ Etudes Nucleaires de Bordeaux Gradignan, France.
INTRODUCTION
Since oxygen radicals have been implicated in the genotoxic events which can initiate and promote cancer development (for review: Ames et al., 1995), numerous clinical trials have been carried out involving anticarcinogenic effects of natural or synthetic antioxidants (for review: German, 1995). Moreover, experimental and clinical studies have also indicated that some natural antioxidant enhance therapeutic treatment of established carcinomas. Mechanisms for antitumor activity of antioxidant compounds generally involve cell differentiation and stimulation of the immunological defense. Recent studies have also revealed some potentiality for direct inhibition of cell growth.
Numerous experimental results are consistent with the assumption that the cellular environment becomes more prooxidizing during differentiation (Allen, 1991; Nagy et al., 1993; Nagy et al., 1995). Generally, it is assumed that antioxidants are inhibitory to differentiation in many types of cells (Takenaga et al., 1981; Allen and Verkatraj, 1992). However, natural retinoids and carotenoids have inhibited the growth of several tumors in vivo due to increasing the rate of malignant cells (for review: Cornic et al., 1994; Bertram Bortkiewicz, 1995). Consequently, all trans-retinoic acid has been used with success in clinical trials, allowing complete remission in acute promyelocytic leukemia patients (Warell et al., 1991). Differentiation is mediated by nuclear retinoid receptor which play an important role in the control of gene expression (Pfhal et al., 1994)
Interestingly, natural antioxidants such as d-limonene, β-carotene, canthaxanthin, and α-tocopherol have caused regression of tumors, exhibiting similarities in anticancer responses with certain chemotherapeutic agents (Elegbede et al., 1986; Shklar et al., 1987; Schwartz and Shklar, 1988; Schwartz et al., 1989; Shklar et al., 1989; Schwartz and Shklar, 1992). For example α-tocopherol inhibits tumor cell growth through a process that reduces free radical production (Schwartz et al., 1993). α-tocopherol bond to membrane associated proteins inhibits the development of peroxidation products. The reduction of peroxidation products directly alter induction of transcription factors such as c-fos and c-myb, genetic products that promote the proliferation of tumor cells (Amstad et al., 1990; Stoler, 1991). Available evidence also indicates that α-tocopherol inhibits the expression of mutant p53, while increasing the expression of wild type form (Schwartz et al., 1993). α-tocopherol may regulate phospholipid products, ultimately modifying calcium flux and membrane kinase activity. A reduction in kinase activity may affect cyclin activity enhancing antioncogene expression such as p53.
Therefore, strong indications support that natural antioxidants can exhibit antiproliferative activity and would probably yield to new drugs in cancer therapy. Nevertheless, high doses of antioxidant compounds were generally required to obtain cytostatic activity in vitro, and cause toxic side effects in vivo. Thence, the search for natural antioxidants with low toxicity and high free radical quenching activity is of critical interest. In the present study, we have investigated the biological action and toxicity of the natural free radical scavenger Bio-normalizer (Santiago et al 199, Santiago et al 1992a)
Among the different analysis performed, cell proliferation assays have been carried out on cisplatin-sensitive and resistant human ovarian adenocarcinoma cells, in presence of Bio-Normalizer alone or in combination with two anticancer agents (cisplatin and melphalan). As a matter of fact, recent studies suggest that antioxidant mau also found a role in conjunction with cytotoxic chemotherapy. Several natural antioxidant compounds have been used to limit the toxicity due to prooxidant effect of anticancer drugs (Sunstorm et al., 1989, Bienvenue et al., 1992; Stahelin, 1993; Vasavi et al., 1994). Furthermore, other studies have demonstrated that antioxidants can also enhance the effectiveness of anti tumor drugs by increasing the sensitivity of tumor cells to these drugs. For example β-carotene and α-tocopherol were able to potentiate the activity of anticancer drugs such as cisplatin and melphalan (Schwartz et al., 1992, Teicher et al., 1994). This activity could be of particular interest in order to overcome clinical resistance to platinum or alkylating agents.
1.1 MATERIAL AND METHODS
1.1. Reagents and Drugs. Owen’s reagent, 3-(4,dimethyltiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfopheny)2-H tetrazolium (MTS), and phenazine methosulfate (PMS) were purchased from Promega, melphalan from Sigma Chemical Co., and cisdiamminedichloroplatinum (II) cisplatin from Laboratoires Roger Bellon.
1.2 Cell Lines. Two experimental cellular models were used in this study. IGROVI human ovarian adenocarcinoma cells grow as monolayer in RPMI 1640 medium supplemented with antibiotics and 10% fetal calf serum. This cell line exhibits an epithelial character, highly tumorigenic properties and a 24H doubling time (Benard et al 1985). A cisplatin-resistant subpopulation, IGROVI-DDP, was established by stepwise escalation with increasing concentrations of cisplatin in the growth medium. Resistant cells were adapted to grow in presence of 1 µg/ml cisplatin. The selection of cisplatin-resistant cells led to a cytologically heterogenous cell population. Two morphological types coexist; small mononuclear cells, morphologically similar to parental IGROVI cells, and enlarged polynuclear cells with heterogeneous karyotype characteristics. IGROVI-DDP cells, when exposed continuously to 1 µg/ml cisplatin, have an average cell cycle time of 66 h. Growth conditions were at 37°C in an humidified atmosphere of 5% (v/v) CO2 in air. Cells were passaged when they reached confluence by treatment with trypsin/EDTA solutions (0.0510.02%), with a transfer factor of ¼.
1.3 Cell proliferation assays. For these experiments, IGROVI and IGROVI-DDP cells were grown in plastic 96-well microtiter plates. Cells in exponentially growth were exposed to various concentrations of Bio-Normalizer, cisplatin or melphalan alone, or in combination. Incubation times were of 2h for cisplatin and melphalan and 2 or 24 h for Bio-Normalizer. After drug exposure, a recovery period of 48h for IGROVI and 72h for IGROVI-DDP cells was performed, then cells were incubated during 2h with 333 µg/ml MTS and 25uM PMS. Solutions of reagents and drugs were prepared just prior to addition to assay plates. MTS is bioreduced by cells into a formazan by dehydrogenase enzymes found in metabolically active cells. The quantity of formazan produced is directly proportional to the number of living cells in culture. After 4h reaction, the absorbance of the formazan was measured at 490 nm with a Bio-Tek Instruments EL 307 microplate reader. 12 wells were analyzed for each conditions of drug exposure in order to obtain a significant mean value of the absorbance. The statistical significance of differences between groups was assessed by the Student’s t-test for paired samples (P<0.05).
1.4 Chemical Analysis: Retinoids, β-carotene and α-tocopherol, in 10 mg/1 Bio-Normalizer solutions in K2HPO4 (5 mM, pH 8), were simultaneously quantified by liquid chromatography (HPLC). The HPLC method was a modification of that described previously (Simonoff et al., 1992). Absorbances of α-tocopherol, retinoids and β-carotene were measured respectively at 292, 325 and 436 nm, Selenium content of Bio-Normalizer was analyzed by particle induced X-ray emission (PIXE). using yttrium as internal standard. The methodology for PIXE analysis in food and biological samples has been described in (Simonoff et al., 1988). Fatty acids in BioNormalizer lipid extracts were measured by gas chromatography. Methods of extraction and quantification of fatty acids were based on those described by (Lepage & Roy, 1986).
1.5 Cytololgical observation. IGROVI and IGROVI-DDP cells were incubated during two weeks in presence of 4 mg/ml of Bio-Normalizer in complete culture medium. Cell morphology, was examined during this high dose incubation by phase contrast light microscopy. Video images of the characteristics cell types of both cell lines were, taken to be compared with controls. Growth characteristics (doubling times) were also controlled
1.6 Nuclear Micro-probe Analysis. NMPA was accomplished using the CENBG nuclear microprobe. The features of device and its capabilities for biological samples analysis have been previously reported (Labador et al., 1990; Moretto et al., 1993). The methodology for specimen preparation of IGROVI cells after 5h incubation with 10 µg/ml cisplatin alone, or in combination with 1000 µg/ml Bio-Normalizer. Large square areas (50×500 µm 2) were irradiated to asses the average metal composition of each cell line. Samples were irradiated with a 1.0 nA beam of 2.5 MeV protons focused to a 5 µm spot. Irradiation times of nearby 5 h were necessary for trace metal determination. High speed scans (1 ms per step) were performed to avoid thermal damage. Simultaneous measurements of particle induced X-rays and backscattered protons were achieved. PIXE spectra were treated with the GUPIX software (Maxwell et al., 1989). Backscattered particles were collected with a 20 mm2 silicon detector, at a scattering angle of 135°). The backscattering data were processed using an in house computer software, specifically designed for the treatment of spectra from biological samples (Moretto & Razafindrabe, 1995).
II. RESULTS
11.1. Cell Proliferation Assays
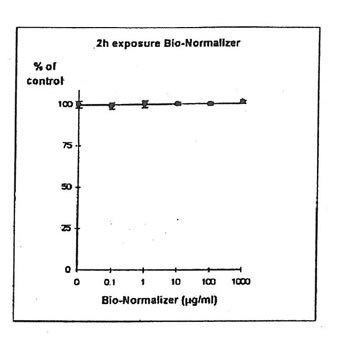
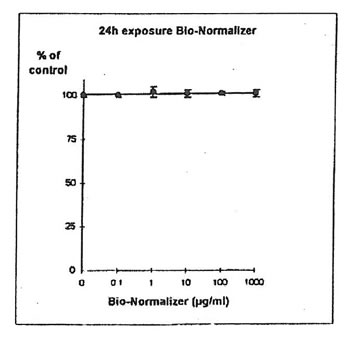
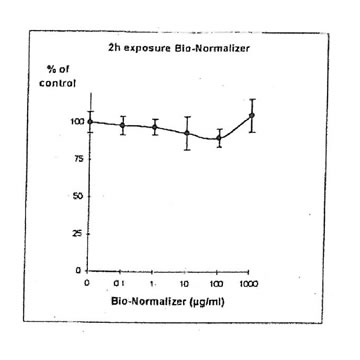
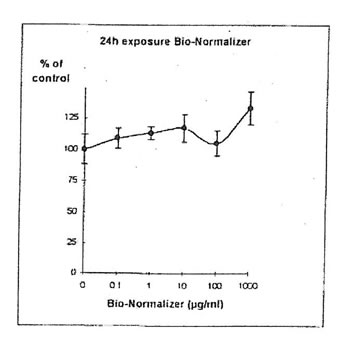
11.1.a. Bio-Normalizer alone. IGROVI and IGROVI-DDP cells were incubated with various concentrations of Bio-Normalizer 0, 0.1, 1.0, 10, 100 and 1000 µg/ml. At these concentrations, no modification of IGROVI cell proliferation could be noticed after 2h exposure (Fig. 1), or after 24h exposure (Fig. 2). In the case of IGROVI-DDP cells, after 2h exposure, proliferation was virtually not modified except a slight enhancement at 1000 µg/ml (Fig 3). After 24h incubation, IGROVI-DDP cell proliferation was slightly enhanced even at low concentrations of Bio-Normalizer (Fig 4).
Figure 1. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (expressed as % of control). After a short treatment (2h)
Figure 2. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (expressed as % of control), after a long treatment (24h)
Figure 3, Effects of different concentrations of Bio-Normalizer on IGROVI-DDP cells growth (expressed as % control), after a short treatment (2h)
Figure 4. Effects of different concentrations of Bio-Normalizer on IGROVI-DDP cell growth (expressed as % control), after a long treatment (24h.
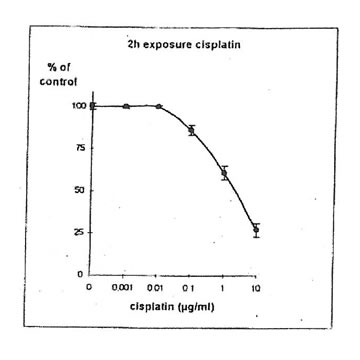
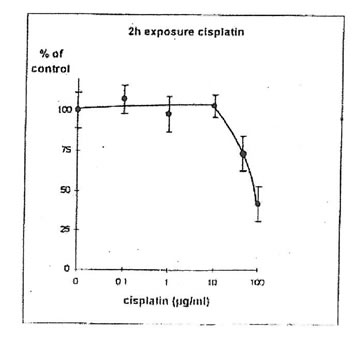
11.1. b. Determination of cisplatin IC50. Cisplatin concentrations inhibiting 50% of cell proliferation (IC50) were determined both on IGROVI and IGROVI-DDP, after 2h of drug exposure (Fig. 5 and 6): for IGROVI, IC50 = 1 µg/ml cisplatin; for IGROVI-DDP, IC50 = 85 µg/ml cisplatin.
Figure 5. Effects of different concentrations of cisplatin on IGROVI cell growth (expressed as of control), after 2h exposure
Figure 6. Effects of different concentrations of cisplatin on IGROVI-DDP cell growth
(Expressed as % of control, after 2h exposure.
11.1.c. Bio-Normalizer and cisplatin IGROVI AND IGROVI-DDP cells were incubated During 2h with their respective IC50 concentrations of cisplatin, and simultaneously with various concentrations of Bio-Normalizer (0, 0.1, 1.0, 10, 100, 1000 µg/ml), or pre-incubated cells, cisplatin cytotoxicity was partially suppressed by Bio-Normalizer, even at low concentrations of Bio-Normalizer (0.1 µg/m1) (Fig 7). Cell proliferation varied between 74% (at 1 µg/ml Bio-Normalizer) and 80% (at 1000 µg/ml Bio-Normalizer), instead of 50% normally observed for a 2h exposure with 1µg/ml cisplatin without Bio-Normalizer. The same phenomenon was observed, and amplified, when IGROVI cells were pre-exposed during 24h to several concentrations of Bio-Normalizer (Fig. 8). In this case, cell proliferation reached 88% to 96% of control.
For IGROVI-DDP cells, after a short incubation with Bio-Normalizer, cisplatin cytotoxicity, measured after 2h- exposure with 85 µg/ml cisplatin, was totally suppressed. Cell proliferation compared to controls varied between 92 and 111%, instead of 50% without Bio Normalizer (Fig. 9). The same was observed after 24h pre-exposure of IGROVI-DDP cells with Bio-Normalizer; cisplatin cytotoxicity was nearly totally suppressed with cell proliferation rates between 82 and 100% instead of 50% (Fig. 10).
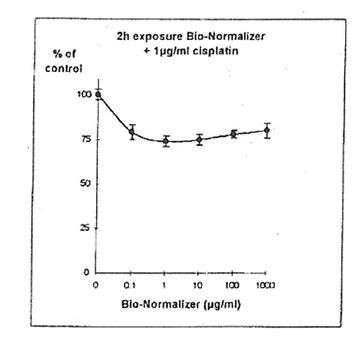 Figure 7. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (expressed as % of control), after 2h with simultaneous exposure to 1 ug/ml cisplatin.
Figure 7. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (expressed as % of control), after 2h with simultaneous exposure to 1 ug/ml cisplatin.
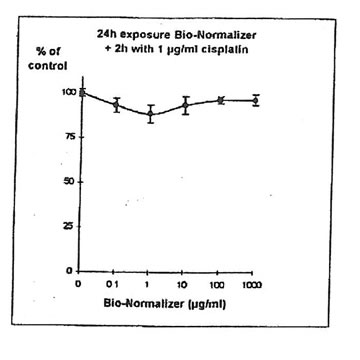 Figure 8. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (expressed as % of control), after 24h pre-treatment and 2h exposure to 1 µg/ml cisplatin.
Figure 8. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (expressed as % of control), after 24h pre-treatment and 2h exposure to 1 µg/ml cisplatin.
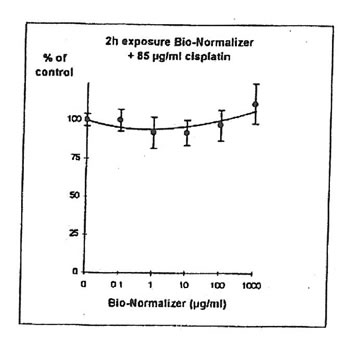 Figure 9. Effects of different concentrations of Bio-Normalizer on IGROVI-DDP cell growth (Expressed as % of control), after 2h with simultaneous exposure to 85 µg/ml cisplatin.
Figure 9. Effects of different concentrations of Bio-Normalizer on IGROVI-DDP cell growth (Expressed as % of control), after 2h with simultaneous exposure to 85 µg/ml cisplatin.
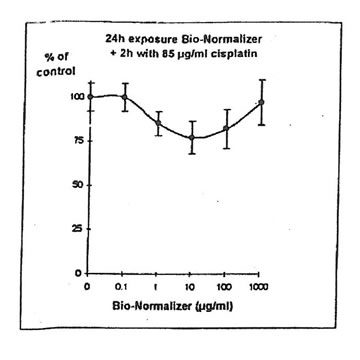 Figure 10. Effects of different concentrations of Bio-Normalizer on IGROVI-DDP cell growth (expressed as % control), after 24h pre-treatment and 2h exposure to 85 µg/ml cisplatin.
Figure 10. Effects of different concentrations of Bio-Normalizer on IGROVI-DDP cell growth (expressed as % control), after 24h pre-treatment and 2h exposure to 85 µg/ml cisplatin.
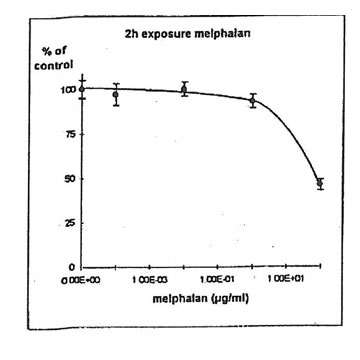
11.1.d. Determination of melphalanlC50. Melphalan concentration inhibiting 50% of cell proliferation (IC50) was determined on IGROVI cells, after 2h drug exposure (Fig. 11). For IGROVI, IC50 = 95 µg/ml melphalan.
Figure 11. Effects of different concentrations of melphalan on IGROVI cell growth (expressed as % of control), after 2h exposure.
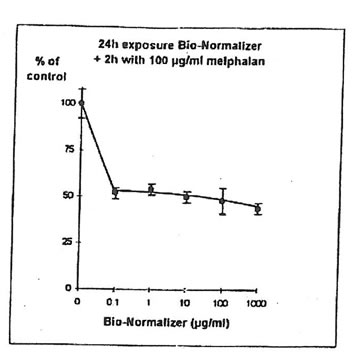
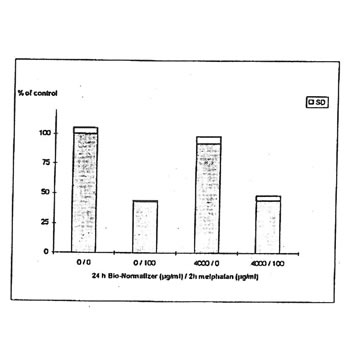
11.1. e. Bio-Normalizer and melphalan. IGROVI cells were incubated during 2h with 100 µg/ml melphalan, and simultaneously with various concentrations of Bio-Normalizer (0, 0.1, 1.0, 10, 100, 1000 µg/ml), or pre-incubated with these concentrations of Bio-Normalizer during 24h. During simultaneous exposure with Bio-Normalizer and melphalan, the resultant cytostatic activity of melphalan was significantly modified (Student’s t-test P< 0.15) (Fig. 12). Indeed, a moderate enhancement of cytotoxicity was noticed with a cell proliferation rate ranging from 33 ± 7 % to 40 ± 5 % when cells were simultaneously exposed to 100 µg/ml and various concentrations of Bio-Normalizer. In comparison, the corresponding value was 46 ± 3% when cells were exposed only to 100 µg/ml melphalan. However, melphalan cytotoxicity was not modified after 24 h pre-incubation with various concentrations of Bio-Normalizer. In this case, cell proliferation varied between 44±3 and 54 ± 3% for melphalan and Bio-Normalizer combinations (46 ± 3% for Melphalan alone) (Fig. 13). This observation was confirmed by long treatment with high doses of Bio-Normalizer (Fig. 14). Effectively, A 24h pre-incubation of IGROVI cells with 400 µg/ml of Bio-Normalizer in the culture medium did not change cell proliferation rates after 2 h exposure to 100 µg/ml melphalan.
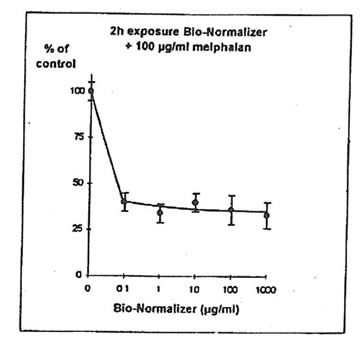 Figure 12. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (expressed as % of control), after 2h with simultaneous exposure to 100 µg/ml melphalan.
Figure 12. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (expressed as % of control), after 2h with simultaneous exposure to 100 µg/ml melphalan.
Figure 13. Effects of different concentrations of Bio-Normalizer on IGROVI cell growth (Expressed as % of control), after 24h pre-treatment and 2h exposure to 100 µg/ml melphalan.
Figure 14. Effects of Bio-Normalizer (4000 ,ug/ml) on IGROVI cell growth (expressed as % of control), after 24h pre-treatment with without 2h exposure to 100 µg/ml melphalan.
11.2. Chemical Analysis
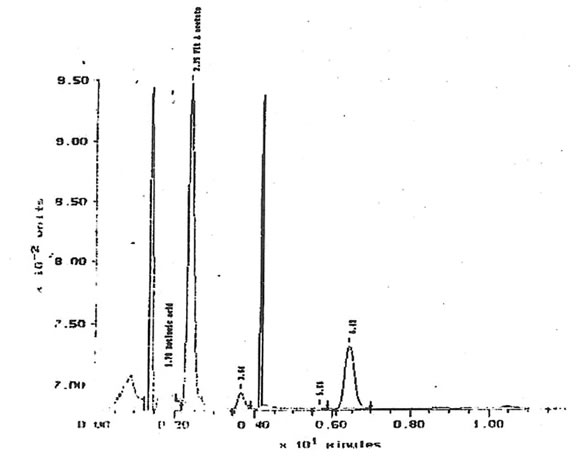
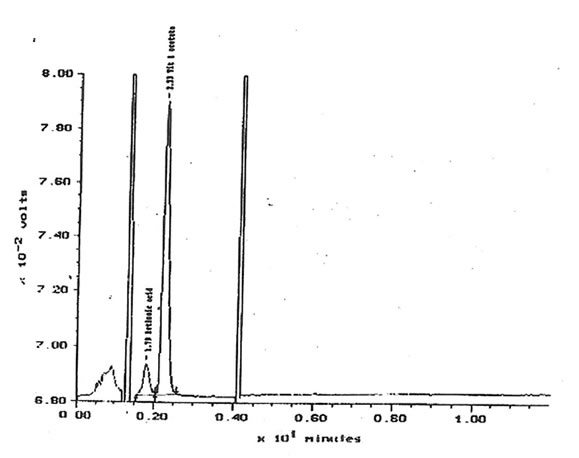
11.2.a. Retinoids, β-carotene and α-tocopherol. The quantities of retinol, β-carotene and α-tocopherol extracted from solutions of 10 µg/1 Bio-Normalizer in 5 mM K2HPO4 were under the limit of detection. Respective limits of detection for retinol, β -carotene and α-tocopherol were approximately 500, 100 and 3600 µg/g of Bio-Normalizer. Retinoic acid has been detected within lipid extracts at 752± 13 µg/g of Bio-Normalizer. Characteristic chromatogram corresponding to HPLC analysis of Bio-Normalizer extracts from 10 mg/ml solutions in K2HPO4, and to control solutions with standards of retinoic acid and retinyl acetate are presented in Fig. 15 & 16.
Figure 15, Chromatogram of Bio-Normalizer solution lipid extracts with retinyl acetate and tocopherol acetate internal standards. Peak at 1.78 min = retinoic acid, 2.35 min= retinyl acetate, 6.42 min = tocopherol acetate.
Figure 16. Control chromatogram with retinoic acid and retinyl acetate internal standards.
Peak at 1.79 min. = retinoic acid, 2.23 min = retinyl acetate
11.2b. Selenium. Selenium content of Bio-Normalizer was inferior to 1µg/g limit of detection obtained after pre-concentration and PIXE analysis.
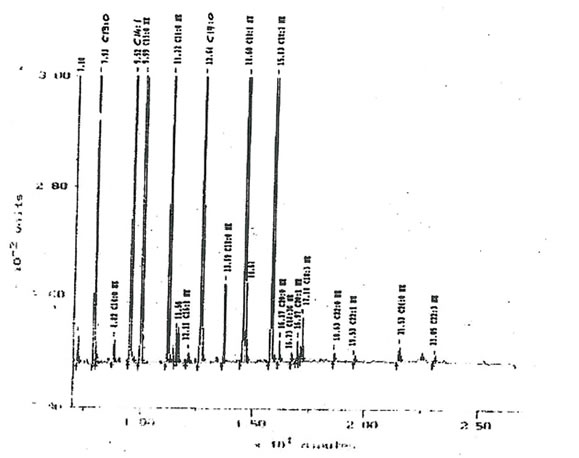
11.2.c. Fatty acids. The total mass of fatty acids in Bio-Normalizer is relatively low (inferior to 0.1%). The main forms encountered in Bio-Normalizer lipid extracts were C13:0, C14:1, C16:0, C17.0, C18:1, C18:2 and C18:3a (Fig. 17). Their quantity within Bio-Normalizer ranged from approximately 10 µg/g (dry mass), for C18:0 and C18:3 α., to 150 µg/g for C14:1 and C18:2. This composition is classical of plant extracts.
Figure 17. A typical chromatogram offatty acids distribution within Bio-Normalizer lipid extracts. The main components detected are C13: 0, C14:1, C 16, C 17: 0, C18: 0, C18:1, C18:2
and C.18:3 α
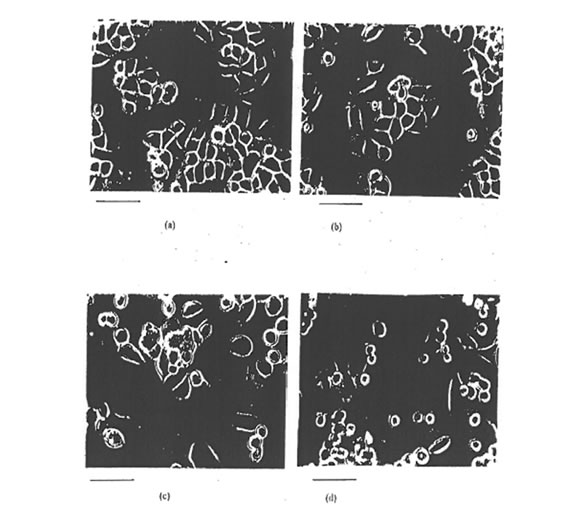
11.3. Cytological observations. After two weeks incubation with 4 µg/ml Bio-Normalizer, no modification of cellular morphology could be noticed on IGROVI, or on IGROVI-DDP cells (Figure 18). However, a slight increase in the growth rate of cisplatin-resistant cells was remarked, whereas IGROVI growth remained unchanged. Effectively, when IGROVI-DDP cells were exposed continuously to 1 µg/ml cisplatin and 4 mg/ml Bio-Normalizer, passages occurred after 7 days instead of 9 days. A decreased efficacy in cell separation with trypsin/EDTA solutions was also noticed during continuous incubation with Bio-Normalizer.
Figure 18. Phase contrast light microscopy video images of IGROVI cells (a, b) and IGROVI -DDP cells (c, d), cultured with Bio-Normalizer free medium (a, c), or with 4 m/ml Bio-Normalizer in complete medium (b, d); bar, 50 µm.
11.4 Nuclear Micro-Probe Analysis. Essential trace metal (Mn, Fe, Cu, Zn) and platinum were quantified in IGROVI cells after 5h incubation with 10 µg/ml cisplatin with, or without, 1000 µg/ml Bio-Normalizer (Tabel 1). Only one analysis could be carried out on cells exposed to Bio- Normalizer, therefore these results must be considered as preliminary and confrimed by further analysis. According to this first experiment, platinum uptake in IGROVI cells appeared to be reduced by the action of Bio-Normalizer with an approximate decreased of 75% of incorporation.
Table 1. Platinum and trace metal concentrations (pglg dry mass) in 1GROVI cells with/without
5 h exposure to 10 pglm/ cisp/atin, and to 10 pglm/ cisp/atin + 1 mg/ml Rio-Normalizer.
|
Cellular Content (µg/g dry mass) |
IGROVIa |
IGROVIa 10 µg/ml cisplatin |
IGROVIa 10µg/ml cisplatin 1µg/ml Bio-Normalizer |
|
Mn |
9.5 ± 1.6 |
12. 7 ± 7.0 |
11.2 ±1.0 |
|
Fe |
143 ± 19 |
167 ± 11 |
126 ± 2 |
|
Cu |
11.7 ± 3.8 |
10.5 ± 1.3 |
7.8 ± 0.8 |
|
Zn |
212 ± 23 |
252 ± 53 |
187 ± 3 |
|
Pt |
|
283 ± 8 |
66.6 ± 7.0 |
a.b; n = 5, mean ± SD.
c: n = 1, concentration± error
Scanning dimensions: 500x 500 µm2 ( corresponding to nearly 500 IGROVI cells).
DISCUSSION
Bio-Normalizer at concentrations ranging from 0.1 to 1000 µg/ml have demonstrated no inhibition of cell proliferation on IGROVI OR IGROVI-DDP cells, after a short or a long treatment (2 or 24 hours exposure). This observations suggest that Bio-Normalizer alone does not present any toxicity at pharmacological or supra-pharmacological concentrations on IGROVI or IGROVI-DDP cells. This lack of toxicity was confirmed by long incubations (2 weeks), of IGROVI cells with high doses of Bio-Normalizer ( 4 mg/ml). In these conditions, no changes were apparent on IGROVI cell morphology and growth. These results corroborate the fact that
Bio-Normalizer is a natural non-toxic nutritional health food (Santiago et al., 1992b).
However, several other studies have demonstrated that some natural antioxidants can exhibit antiproliferative activity (Elegbede et al., 1986; Shklar et al., 1987; Schwartz & Shklar,
1988; Schwartz et al., 1989; Shklar et al.,1989; Schwartz & Shklar, 1992); It was hypothesized
that antioxidants inhibit tumor cell growth through a 95% air, inhibition of tumor cell survival
would required very high levels of antioxidants (Schwartz et al., process that reduces free radical
production. Therefore, in vitro inhibition of growth is a function of the oxygen state of radical, production. Therefore, in vitro inhibition of growth is a function of the oxygen state of the tumor cell target. A normal oxygen pressure like in these experiments 5% CO2., 1993) Thus, it would be particularly important to determine Bio-Normalizer antiproliferative activity on cancer cells in
an oxygen-restricted ervironment. As a matter of fact the level of reactive molecules should then
be controlled by Bio-Normalizer free radical scavenger components, resulting in a decreased expression of transcription factors and membrane kinase activity, and ultimately a decreased cell
proliferation. This outcome would represent a fundamental breakthrough as regions of low oxygen (hypoxia) are very common features of solid tumors (Vaupel & Rockel, 1995).
Cisplatin-resistant cells, IGROVI-DDP, exhibited an increased growth rate when incubated with Bio-Normalizer. This phenomenon appeared in short treatment assays at high doses (1000 µg/ml), and was more clearly noticed after long treatments, even at low doses of Bio-Normalizer (0.1 µg/ml). These observations may explain the increased growth rate of IGROVI-DDP cells noticed during continuous exposure with 4 mg/ml Bio-Normalizer. As IGROVI-DDP cells are cultivated in presence of 1 µg/ml cisplatin, in order to maintain cisplatin resistant phenotype, it can be hypothesized that Bio-Normalizer interacts with cisplatin, and decreased its cytostatic effect. Cell proliferation assays with combinations of cisplatin and Bio-Normalizer supported this hypothesis. Simultaneous exposure of IGROVI cells to 1 µg/ml cisplatin during 2h (IC50), and 0.1 to 1000 µg/ml Bio-Normalizer resulted only in 25% inhibition of cell proliferation. During long treatments, this effect was enhanced and the maximum inhibition was only 12%, with 10 µg/ml cisplatin and 10 µg/ml Bio-Normalizer, instead of 50% expected when cisplatin was administered alone. The same was true for the cisplatin resistant cell line IGROVI-DDP. For this cell line, IC50 corresponded to 2h exposure with 85 µg/ml cisplatin. Simultaneous exposure of IGROVI-DDP cells with 85 µg/ml cisplatin and Bio-Normalizer (0.1 to 1000 µg/ml) during 2 hours resulted in a nearly total inhibition of cisplatin antiprolierative effects. Similarly, during long treatments, the inhibitory effect of Bio-Normalizer on Cisplatin cytotoxicity was also remarked. Therefore, it can be concluded that Bio-Normalizer interferes in vitro with cisplatin pharmacology, even at low doses, and prevent its cytotoxic effects.
Additionally, preliminary nuclear probe microanalysis results suggested that Bio-Normalizer interacts with cisplatin in the extrcellular environment. Effectively, reduced intracellular platinum concentrations were measured in cultured cells after simultaneous exposure with cisplatin and Bio-Normalizer. Protection against organoplatinum toxicity, and more generally against heavy metals toxicity, could represent an unexpected application of Bio-Normalizer. It would result of particular interest to continue these analysis in order to confirm the first observation. Effectively, this property would support the efficacy of Bio-Normalizer as a
natural health food, enlarging its domain of action to the protection against heavy metals. Moreover, in the case of a strong interaction between cisplatin and Bio-Normalizer involving stable metal-binding, platinum would represent an exogenous tracer that would facilitate the identification of Bio-Normalizer active components.
Chemical analysis of Bio-Normalizer revealed that among the natural antioxidants analyzed such as retitioids, β-carotene, α-tocopherol, or selenoproteins, only retinoic acid was detected. The concentration measured (750 µg/g) is theoretically able to induce retinoic acid associated effects such as activation of cell differentiation. However, IGROVI cell proliferation
is not affected by retinoic acid, even at high concentration (Caliaro et al., 1994). Therefore, another cellular model should be used to test pro-differentiative effects of Bio-Normalizer.
After 2h of simultaneous treatment with Bio-Normalizer and melphalan, a slight, but significant, enhancement in melphalan cytostatic effect was noticed. This phenomenon was remarked even at low concentrations of Bio-Normalizer (0.1 µg/ml ). The maximum effect was
measured at 1000 µg/ml Bio-Normalizer and corresponded to 28% enhancement of growth inhibition. However, IGROVI cell proliferation, after melphalan exposure, was unchanged when
pre-incubation with various concentrations of Bio-Normalizer were performed. Therefore, further studies seem necessary to confirm the potentiation of melphalan antiproliferative activity by Bio-Normalizer.
CONCLUSION- PERSPECTIVES
In this study, we have investigated whether the natural free radical scavenger, Bio-Normalizer, could inhibit in vitro human ovarian carcinoma cells proliferation, and modulate cisplatin of melphalan anticancer activity.
We have demonstrated that Bio-Normalizer exhibits no cytotoxicity on human ovarian. adenocarcinoma cells, even at supra-pharmacological concentrations. This lack of toxicity may
represent very positive characteristics if the hypothesis of anticancer activity through reduction of free radical production is confirmed in hypoxic conditions. It is now important to complete the investigation of Bio-Normalizer anticancer capabilities by cell proliferation assays in oxygen restricted conditions. This additional experiments would permit to conclude definitely on the potential role of Bio-Normalizer for inhibition of tumor cell growth.
Bio-Normalizer contains retinoic acid, and maybe other unidentified differentiative compounds, in pharmacological quantity. Differentiation studies should be carried on with adequate protocols and cellular models.
Unexpected interaction of Bio-Normalizer with cisplatin pharmacology results in the inhibition of its cytotoxic action, Therefore, Bio-Normalizer is not able to reverse cisplatin cellular resistance but demonstrates heavy metal protective capabilities. It would result very interesting to identify the mechanism involved in this interference. Preliminary experiments, by
nuclear prove microanalysis, on cells incubated with Bio-Normalizer and cisplatin seemed to reveal a reduced uptake of platinum. If this result is confirmed, it will mean that the interaction
occurs in the extracellular medium, thus providing evidence for metal chelation properties of Bio-Normalizer. Moreover, the fact this interaction involves platinum would allow an easier identification of the active component(s) responsible for metal binding, and maybe for the free
radical protection, if these two phenomena were linked.
Finally, Bio-Normalizer does not reduce the anticancer activity of alkylating agents such
as melphalan. In contrast, a moderate enhancement of melphalan growth inhibition has been observed following simultaneous incubation with Bio-Normalizer. This result could be of major
interest in order to modulate alkylating agents cytotoxicity, and reverse cellular resistance. Further studies should be performed on alkylating agents sensitive and resistant cell lines in order to determine the conditions for an optimal combination.