| Title | MANUFACTURE OF SOLUBLE BIO-NORMALIZER Technological regulations |
|---|---|
| Year | 1997 |
| Author | Igor Afanas’ev,Natalia Polozova, Anatolii Dorozhko, Elena Ostrachovich |
| Publisher |
MANUFACTURE OF SOLUBLE BIO-NORMALIZER
Technological regulations
Approved :
Professor Vladimir Gunar
Doctor of Chemical Science
Director General of Vitamin Research Institute
MOSCOW, 1997
AUTHORS
Supervisor
Professor Igor Afanas’ev
Head of Laboratory, Ph.D., D.Sci.
Research workers:
Natalia Polozova
Senior Research worker, Ph.D.
Anatolii Dorozhko
Senior Research worker, Ph.D.
Elena Ostrachovich
Senior Research worker, Ph.D.
BN Soluble Application Guideline
| Solution | 5% |
| Preparation | Sterilizer (autoclave) solution at over 200°C (possibly 250°C or above) or expose to γ- irradiation or ultrasound challenge. |
| Infusion | 20 drops/min. Maximum 200 mL per infusion |
| Test | Check blood sugar and others by endocrinologist at least 2 days after the cessation of the infusion. |
| Notes: | Blood sugar level will go up. Watch carefully. After infusion, there may be fever. Observe carefully 2nd, especially after 3rd infusion when there may be immunological reactions.) |
Process of BN Soluble
B N
↓
BN+Water
↓ → → Remove unsoluble matters
BN aqueous solution
↓ ←← Aceton
Water/Acetone/BN
↓ Centrifugation → → Fraction I
Soluble BN
↓ Vacuum dry (30°C)
Fraction II
Principal parameter
Melting point:130-l35°C
pH value of 20% aqueous solution: 4.5 ~ 5.0
Specific rotation:+59.5 ~ 61.0° (1% aqueous solution)
Specific biological activity:Inhibition of luminal-amplified CL produced by zymosan-stimulated rat peritoneal macrophages, I50=8-8.5mg/mL
Solubility: Soluble in water but not in organic solvent
Storage: Stable at least 12 months under no exposure to water and light
| Glucose | Biological activity (higher number stronger) | |
| BN | 90-92% | 1 |
| Fraction I | 85-87 | 2 |
| Fraction II | 80-85 | 3 |
TABLE OF CONTENT
CHAPTER 1 Properties of final product
CHAPTER 2 Chemical scheme of the process
CHAPTER 3 Technological schemes of the process
CHAPTER 4 Raw materials, reagents and products
CHAPTER 5 Equipment
CHAPTER 6 Description of technological process
CHAPTER 7 Information data
CHAPTER 1
PROPERTIES OF FINAL PRODUCT
1.1 Name: Soluble Bio-normalizer (Fraction I)
1.2 Origin: The product is prepared on the basis of Bio-normalizer, a natural Japanese food supplement prepared by the fermentation of Carica papaya (Sun-O International Inc., Gifu, Japan).
1.3 Biological activity: The product is nontoxic substance possessing antioxidant and immunostimulatory activities. It’s supposed area of application is the treatment of patients with diabetes mellitis, hepatitis, and various inflammatory diseases as well as people exposed to environmental and therapeutic irradiation.
1.4 Principal parameters.
1.4.1 Product is a white or yellowish powder.
1.4.2 Melting point 130-135°C.
1.4.3 pH value of 20% aqueous solution 4.5-5.0
1.4.4 Specific rotation +59.5-61.0° (1% aqueous solution)
1.4.5 Specific biological activity: Inhibition of luminol-amplified CL produced by zymosan-stimulated rat peritoneal macrophages, 150 = 8-8.5 mg/mL.
1.4.6 Product is completely soluble in water and insoluble in organic solvents.
1.4.7 Product is stable during 12 months at least. It should be stored under conditions excluding exposure to moisture and light.
CHAPTER 2
CHEMICAL SCHEME OF THE PROCESS
BN water, 20°C →Soluble component + Insoluble sediment
Soluble component acetone/water → Soluble BN (Fr.I) + Fr.II
Soluble BN (Fr.I) is precipitated after the addition of acetone to aqueous solution.
Fr.II is the second active product obtained by the evaporation of acetone-water solution after the separation of Fr.I.
CHAPTER3
TECHNOLOGICAL SCHEME OF THE PROCESS
Dissolution of BN
in water
↓
↓
BN suspension
↓
↓
1 centrifugation ——————————————————–> Insoluble sediment
↓
↓
Aqueous BN solution
↓
↓ +acetone
Precipitation of Fr.I
↓
↓
2 centrifugation ————————-> Water/acetone solution of Fr.II
↓ rinsing with acetone ↓
↓ ↓
wet Fr.I Separation of Fr.II by distillation
↓ drying ↓
↓ ↓
Final product: Soluble BN (Fr.I) wet Fr.II
↓ drying ↓
Final product (Fr.II)
CHAPTER 4
RAW MATERIALS, REAGENTS AND PRODUCTS
Bio-normalizer (BN), a natural Japanese food supplement prepared by the fermentation of
Carica papaya (Sun-O International Inc., Gifu, Japan).
Acetone produced in Russia (TU 6-09-3513-86; OSCH, 99.5% purity).
Distilled water.
CHAPTER 5
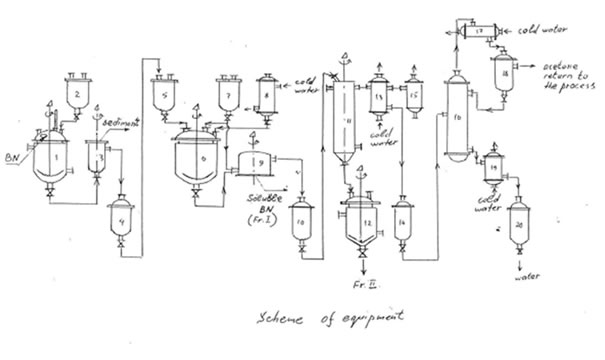
EQUIPMENT
(recommended for manufacturing Soluble BN on a factory)
| N*) | Name | Number | Material | Parameters |
| 1 | Container for BN dissolution | 1 | Stainless steel | Vertical bulk vessel with a jacket and a stirrer |
| 3 | Centrifuge | 1 | Stainless steel | 13500 revolution/min |
| 6 | Container for precipitation of Fr.I | 1 | Stainless steel or enamel | Vertical bulk vessel with a stirrer |
| 9 | Centrifuge | 1 | Stainless steel | Centrifuge with a filter,1500 revolution/min |
| 11 | Apparatus for evaporation of acetone | 1 | Stainless steel | Vessel supplied with a rotor for dispersing the solution in vacuum |
| 16 | Rectification column | 1 | Stainless steel | |
| 12 | Container | 1 | Stainless steel | Vertical bulk vessel |
| 8 | Condenser | 1 | Stainless steel or enamel | Vertical vessel with pipes |
| 13 | Condenser | 1 | Stainless steel | Vertical vessel with pipes |
| 17 | Condenser | 1 | Stainless steel or enamel | Vessel with pipes |
| 19 | Condenser | 1 | Stainless steel | Vertical vessel with pipes |
| 15 | Trap | 1 | Stainless steel or enamel | Vacuum-tight vertical vessel with a cooling jacket |
| 4 | Container | 1 | Stainless steel | Vertical vessel |
| 10,14,18 | Storage tank | 3 | Enameled cast iron | Vertical vessel |
| 20 | Storage tank | 1 | Stainless steel | Vertical vessel |
| 2,7 | Measuring container | 3 | Stainless steel | Vertical vessel |
*) See, the scheme of equipment.
CHAPTER 6
DESCRIPTION OF TECHNOLOGICAL PROCESS
1. Preparation of BN suspension
BN (167.5 g) and distilled water (420 mL) were placed in a glass vessel with a condenser and a stirrer and were stirred for 20 min at 20°C (Table 6.1).
Table 6.1
| N | Product | Loaded (g) |
Loaded (mL) |
Received (g) |
Received (mL) |
| 1 | BN | 167.5 | |||
| 2 | Distilled water | 420 | |||
| 3 | BN suspension | 587.5 | 530 |
2. First centrifugation
BN suspension (530 mL) was centrifuged at 10000 g for 15 min (Table 6.2)
Table 6.2
| N | Product | Loaded (g) |
Loaded (mL) |
Received (g) |
Received (mL) |
| 1 | BN suspension | 587.5 | 530 | ||
| 2 | Aqueous solution of Frs. I and II | 577.3 | 520 | ||
| 3 | Insoluble sediment | 10.2 | |||
| 4 | Total | 587.5 | 587.5 |
3, Precipitation of Fr. I
Aqueous solution of Fr. I and II was placed the glass vessel with a condenser, a stirrer and a separator. After that, an 7.5 excess of acetone was added during 30 min at the continuous stirring of reaction mixture. The sediment (Fr. I) was separated by centrifugation (5000 g, 10 min).
Table 6.3
| N | Product | Loaded (g) |
Loaded (mL) |
Received (g) |
Received (mL) |
| 1 | Aqueous solution of Frs.I and II | 577.3 | 520 | ||
| 2 | Acetone | 3322.3 | 3900 | ||
| 3 | Wet Fr.I (sediment) | 131 | |||
| 4 | Acetone/water solution of Fr.II | 3764.8 | 4610 | ||
| 5 | Losses (Fr.I) | 3.8 | |||
| 6 | Total | 3899.6 | 3899.6 |
4. Drying of Fr. I in vacuum.
Wet Fr. I was dried in vacuum vessel at room temperature for 15 hours (Table 6.4).
Average yield of Soluble BN (Fr. I) is 73.4%.
Table 6.4
| N | Product | Loaded (g) |
Received (g) |
| 1 | Wet Fr .I | 131 | |
| 2 | Dried Fr. I | 123 | |
| 3 | Losses Fr. I Acetone | 0.6 7.4 |
|
| 4 | Total | 131 | 131 |
5. Separation of Fr.II by distillation of acetone/water mixture.
Acetone and water were vacuum-distilled from the acetone/water solution of Fr. II (150 -15 mm Hg) at 35°C, and the wet Fr.II obtained was dried in vacuum vessel at room temperature for 20 hours (Table 6.5). Average) yield of Fr. II is 19.5%.
Table 6.5
| N | Product | Loaded (g) |
Loaded (mL) |
Received (g) |
Received |
| 1 | Acetone/water solution of Fr.II | 3764.8 | 4610 | ||
| 2 | Dried Fr.II | 32.4 | |||
| 3 | Acetone/water Mixture |
3347.5 | 4080 | ||
| 4 | Losses (Fr.II) Acetone Water |
2.1 337.2 45.6 |
|||
| 5 | Total | 3764.8 | 3764.8 |
CHAPTER 7
INFORMATION DATA
Present documentation is the technological regulations of a principally new process of manufacturing the water-soluble fraction (Fr.I) of Bio-normalizer (BN), a natural Japanese food supplement prepared by the fermentation of Carica papaya (Sun-O International Inc., Gifu, Japan). Water-soluble Fr. I possesses all the biochemical activities of BN and, to a certain extent, surpasses it. As a side product, the second fraction (Fr.II) was obtained, which exhibits even greater activities, but the production of this product is not a subject of present documentation.
The developed method of the production of Fr.I includes the following stages:
1. Dissolution of BN in water.
2. Centrifugation of BN suspension and the separation of insoluble sediment.
3. Precipitation by acetone and the separation of Fr. I by centrifugation.
4. Drying of Fr.I.
7.1 Choice of optimal conditions of BN dissolution.
To find optimal conditions of BN dissolution in water, a dependence of the yield of insoluble sediment on temperature and duration of the experiment as well as the amount of water has been studied (Table 7.1).
7.2 Choice of optimal conditions of the sedimentation of Fr.I.
For the isolation of soluble BN fraction the method based on the sedimentation of this product by acetone was chosen. We found that BN is completely insoluble in acetone; therefore, the addition of acetone to aqueous solution of BN ought to result in the sedimentation of water-soluble fraction of BN. It is also known that acetone, which is frequently used as a solvent in manufacturing of drugs and food supplements, does not react with natural organic compounds at room temperature, accessible as a very pure substance, and can be completely removed during the drying stage. It should be noted that the use of other solvents such as ethanol instead of acetone resulted in a very low yield of Fr I (<10%).
It was also shown that an important factor is the sequence of the operations: acetone should be always added to BN solution and not vice versa. When the BN solution was added to acetone, the yield of Fr.I sharply decreased. If needed, centrifugation can be substituted by the filtration of the sediment of Fr. I from solution. However, in this case the losses of acetone enhanced, and its content in the sediment increased that made the subsequent stage of drying more difficult. A dependence of the yield of Fr.I on the amount of acetone added during sedimentation is shown in Table 7.2.
7.3 Optimal conditions of the whole process of the manufacturing Fr.I
On the grounds of the above experiments the following optimal conditions of manufacturing Fr I were chosen:
The amount of water is 2.5 L/1 kg BN.
The duration of dissolution is 20 min.
The temperature of dissolution is 20°C.
Insoluble sediment is cetrifugated at 10000 g.
Fr.I is cetrifugated at 5000 g.
The ratio of acetone/EN solution is 7.5:1
Fr.I is dried in vacuum at room temperature for 20 hours.
To show the reproduction of these conditions, a series of 5 experiments was carried out (Table 7.3). It is seen that the average yield of soluble BN (Fraction I) is equal to (73.4±0.6l)%
Table 7.1
| N of
Exp. |
Amount of water/
1 g BN (mL) |
Temperature
|
Duration
(min) |
Amount of sediment(%)
|
| 1 | 1.0 | 20 | 20 | 1.05 |
| 2 | 2.0 | 20 | 20 | 2.35 |
| 3 | 3.0 | 20 | 20 | 2.45 |
| 4 | 4.0 | 20 | 20 | 2.44 |
| 5 | 2.5 | 16 | 20 | 1.80 |
| 6 | 2.5 | 20 | 20 | 2.45 |
| 7 | 2.5 | 25 | 20 | 2.47 |
| 8 | 2.5 | 30 | 20 | 2.45 |
| 9 | 2.5 | 20 | 10 | 1.2 |
| 10 | 2.5 | 20 | 15 | 2.1 |
| 11 | 2.5 | 20 | 20 | 2.45 |
| 12 | 2.5 | 20 | 25 | 2.45 |
Table 7.2
Dependence of the yield of Fr. I on the ratio of acetone/aqueous BN solution.
| N of exp. | Ratio of acetone/aqueous BN solution (v/v) |
Yield of Fr.I (%) |
| 1 | 3.5 | – |
| 2 | 5.0 | 10 |
| 3 | 6.5 | 55.3 |
| 4 | 7.5 | 73.4 |
| 5 | 9.0 | 73.5 |
Table 7.3
Preparation of Fr. I under optimal conditions
| N | Temp of dissolution (°C) |
Duration of dissolution min |
BN (g) Loaded |
|||||||
| 1 | 20 | 20 | 167.0 | 417 | 3900 | 4.08 | 529 | 4550 | 122 | 73.4 |
| 2 | 20 | 20 | 167.5 | 420 | 3900 | 4.15 | 520 | 4610 | 122 | 72.8 |
| 3 | 20 | 20 | 168.3 | 420 | 4000 | 4.05 | 525 | 4630 | 124 | 73.7 |
| 4 | 20 | 20 | 165.5 | 415 | 3800 | 4.13 | 515 | 4580 | 122.5 | 74..0 |
| 5 | 20 | 20 | 168.5 | 425 | 3900 | 4.05 | 520 | 4630 | 123.5 | 73.3 |
| Mean | 20 | 20 | 167.4±0.7 | 420±5 | 3900±100 | 4.10±0.04 | 520±7 | 4610±60 | 123±1 | 73.4±0.6 |
CHROMATOGRAPHIC ANALYSES OF
BIONORMALIZER (powder) and SOLUBLE BN
THIN-LAYER CHROMATOGRAPHY (TLC)
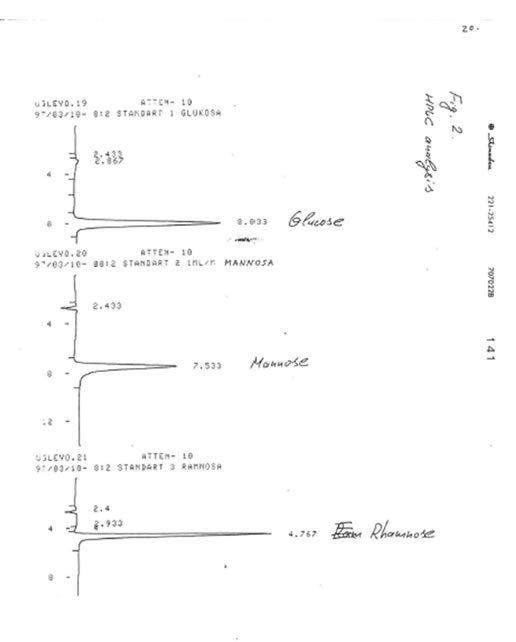
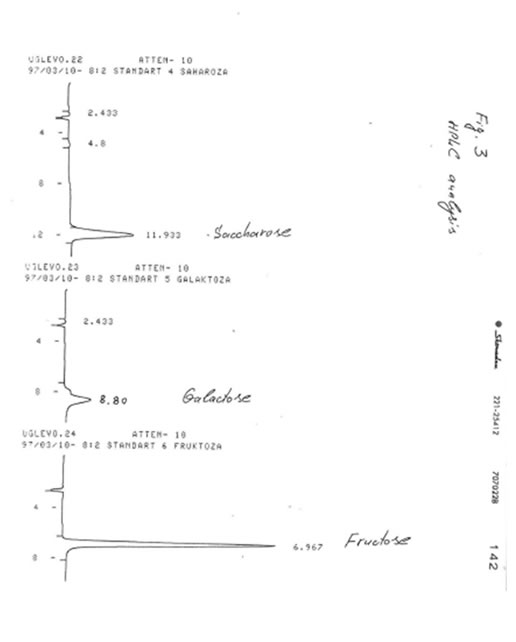
TLC analyses of BN powder, solubles BN (Fr. I), and soluble Fr. II were carried out on the Kieselgel 60 adsorbent in two solevents: solvent A (isopropanol/water, 4:1) and solvent B (normal propanol/ethyl acetate/water, 7:1:2). Samples were applied as 1% solutions. Values of retention times (Rf) for BN samples were compared with Rr values for glucose, mannose, rhamnose, saccharose, galactose, and fructose (Table). It was found that the predominant component of BN powder, soluble BN, and Fr. II is glucose.
Table
| Sample | Rf in solvent A | Rf in solvent B |
| BN (powder) | 0.62 | 0.50 |
| Soluble BN (Fr.I) | 0.62 | 0.50 |
| Fr. II | 0.62 | 0.50 |
| Glucose | 0.62 | 0.50 |
| Mannose | 0.65 | 0.55 |
| Rhamnose | 0.73 | 0.70 |
| Saccharose | 0.58 | 0.43 |
| Galactose | 0.54 | 0.42 |
| Fructose | 0.58 | 0.48 |
HIGH-PRESSURE LIQUID CHROMATOGRAPHY (HPLC)
HPLC analysis is one of the most up-to-date sophisticated analytical methods, which permits to determine the quantitative composition of complex biological mixtures. For analyses of BN samples we used a Knauer chromatograph (Germany) with refractometric detector produced by Kova co. (Czech Republic).
Experimental conditions:
Column of 3.3 x 300 mm; modified silica gel Separon SQX-NH2 (an adsorbent); acetonitrile/water (8:2 and 1:1) mixtures as solvents; the fluid rate of 1 mL/min. Samples were applied as 1% solutions.
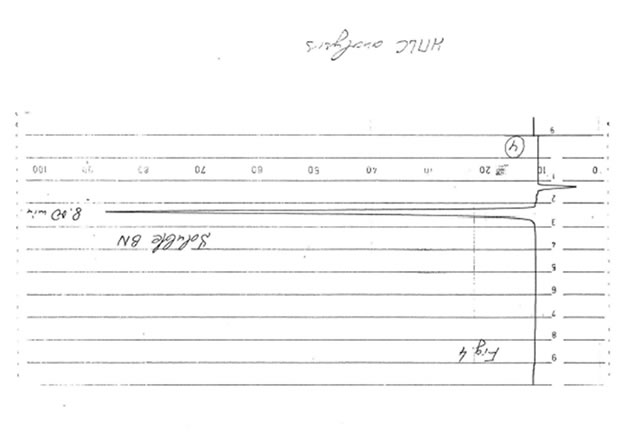
The results obtained are given in Figures 1-3. It was found that in accord with the data of TLC analysis, the predominant component of all samples was glucose. Its retention time (8-8.033 min) completely coincided with that of glucose (8.033 min) and clearly differed from the retention times of mannose (7.533 min), rhamnose (4.767 min), saccharose (11.933 min), galactose (8. 80 min), and fructose (6.967 min). The content of glucose was estimated as 90-92% for BN powder, 85-87% for soluble BN (Fr. I), and 80-85% for Fr. II. As no other peaks were observed on the chromatogram even at the highest level of the chromatograph sensitivity (Fig. 4), we concluded that the other compounds containing in the samples (10-15%) apparently are not carbohydrates (sugars) because the compounds of different structure cannot be analyzed at the same conditions as carbohydrates.
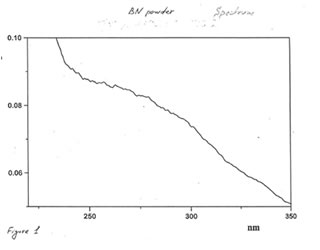
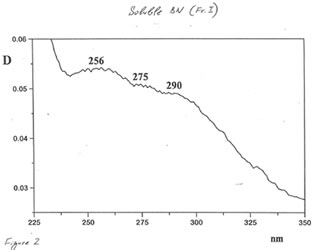
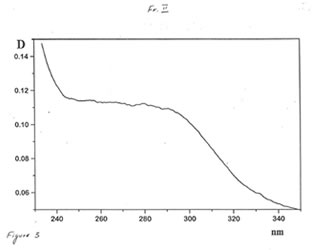
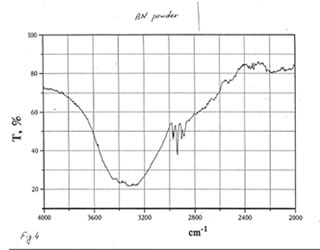
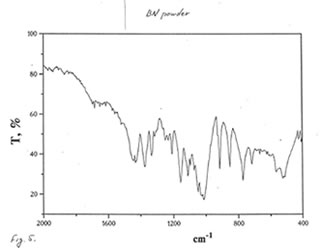
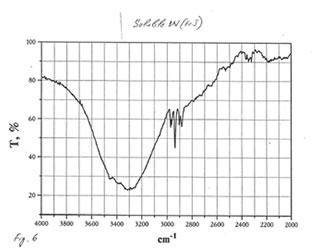
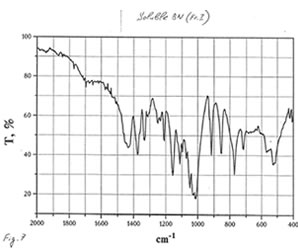
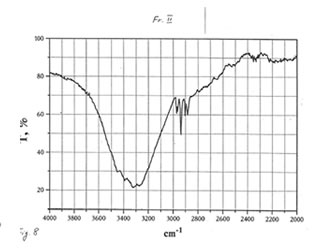
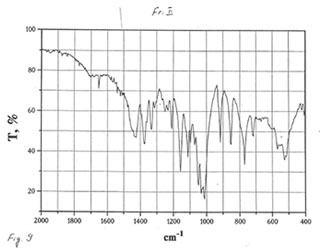
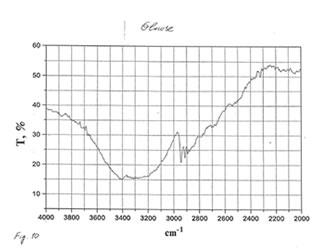
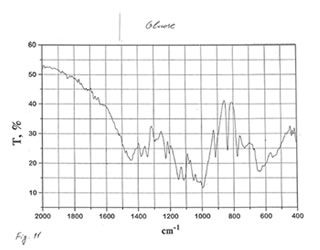
OPTICAL AND IR SPECTRA
Optical and IR spectra were recorded for BN powder, the soluble BN (Fr. I), and Fr. II on a Specord 40 spectrophotometer (Germany) (Figure 1-3). Optical spectra were recorded for aqueous solutions, and IR spectra were obtained in a KBr cuvette. It is seen that all samples absorbed light at wave length λ > 350 nm. It is of interest that the spectrum of the soluble BN contained 3 maxima at 256, 275, and 290 nm (Fig.2), which were absent in the spectra of BN powder and Fr. II. This fact probably indicates the presence some unknown active compounds in the sample of soluble BN.
As one may expect, IR spectra (Figs. 4-9) of all BN samples are similar to that of glucose (Figs. 10, 11). However, the spectra of soluble BN (Figs. 6, 7) and Fr. II (Figs. 8, 9) contain several additional bands at 520 (doublet), 1660 and 2980 (doublet) cm-1 that points out at the presence some unsaturated and nitrogen-contained compounds.
CONCLUSIONS:
Chromatographic and spectral analyses of BN powder, the soluble BN (Fr. I), and Fr. II show that the principal component of all BN samples is glucose. The glucose content decreases from BN powder (90-92%>) to the soluble BN (85-87%), to Fr. II (80-85%). Chromatographic analysis supposes that the other components of BN samples are not carbohydrates (sugars). Spectral analysis indicates the possibility of the presence in the samples of unsaturated and nitrogen-containing compounds.
DETERMINATION OF BIOCHEMICAL ACTIVITY OF SOLUBLE BN
A. ANIMAL EXPERIMENTS
Animals.
Male Wistar rats weighting 150 to 200 g were maintained under standard laboratory conditions, with chow diet and water ad libitum.
Ex vivo studies.
The rats were divided into four groups: group 1 (control 6 animals) received the intraperitoneal injection of 1.0 mL sterile physiological saline; group 2 (6 animals) received the intraperitoneal injection of 10 mg BN powder in 1.0 mL solution; group 3 (7 animal ) received the intraperitoneal injection of 10 mg soluble BN (Fr. I) in 1.0 mL solution; group 4 (6 animals) received the intraperitoneal injection of 10 mg Fr. II in 1.0 mL solution. 24 hours later animals were sacrificed under slight ethyl ether narcosis.
Isolation of peritoneal macrophages.
Peritoneal macrophages were prepared by peritoneal lavage with 2 mL prewanned saline solution. The lavage fluid was filtered and centrifuged at 300 x g for 10 min. Cells were resuspended, twice washed, and stored at 4°C in I-JESS. Macrophage preparations were > 90% pure and > 95% of the cells excluded trypan blue.
Chemiluminescence assays
CL measurements in a Luminometer mod 1251 ( LKB, Sweden) were monitored at 37°C and continuous mixing on a programmed IBM computer. All experiments were carried out in dublicate. Each point was a mean of 3 or 4 independent measurements. Lucigenin-amplified CL was applied as a test for measuring superoxide production and luminal-amplified CL was used as a test on active oxygen species forming during the decomposition of hydrogen peroxide, principally, hydroxyl radicals.
Measurement of lucigenin- and luminal-amplified CL produced by rat peritoneal macrophages. Macrophage suspension (5.0 x 105 cells) and luminal (20 µM) or lucigenin (400 µM) were incubated in HBSS (pH 7.4, a total volume of 1 mL) in the CL unit of a lurninometer for 3 min. After measurement of background for 5 min, CL was activated by adding 0.5 mL (1 mg/mL) opsonized zymosan suspension. An amplitude of the CL response to an activator was defined as the difference between maximal intensity of activated CL and intensity of spontaneous CL.
In vitro studies
Experiments with peritoneal macrophages.
Lucigenin- and luminol-amplified CL produced by rat peritoneal macrophages was measured as in the ex vivo experiments after adding the appropriate concentrations of BN powder, the soluble BN, and Fr. II to the incubation mixture containing macrophage suspension.
Experiments with xanthine oxidase.
Lucigenin (500 µM), xanthine oxidase (30 U), and a BN sample of appropriate concentration were incubated in phosphate buffer (pH 7.6).. The reaction was started by the addition of 100 µM xanthine. After that, the CL intensity was measured.
Statistics
All results are from the experiments carried out in dublicate or triplicates are presented as mean± SD. Differences were analyzed using the Student’s t-test, the level of significance being set at P < 0.05. Each point was a mean of three independent measurements.
RESULTS
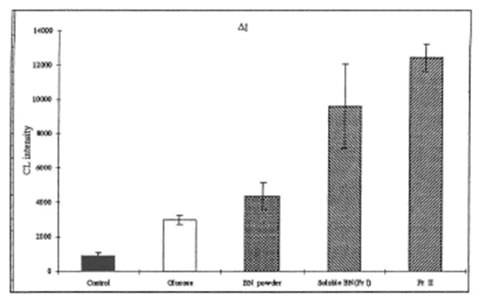
It has earlier been shown by us [ J. A. Osato, I. B. Afanas’ev, Z. P. Cheremisina, T. B. Suslova, N. E. Abramova, E. V. Michalchik, I. B. Deeva, L. A. Santiago, L. G. Korkina, Phys. Chem. Biol. Med. 2, 87-95 (1995); J.A.Osato, L.G.Korkina, E. Michalchik, I.B.Afanas’ev, in Proceedings of the International Symposium on Natural Antioxidants: Molecular Mechanisms and Health Effects, L.Packer, et al., Eds., AOCS Press, 1996, pp. 109-116] that the stimulatory activity of Bio-normalizer can be measured by luminol amplified CL in the ex vivo experiments and its antioxidant activity can be determined in the in vitro experiments. Present data fully confirm this conclusion. As is seen from Fig. 1, the injection of rats with BN powder, soluble BN (Fr. I), and Fr. II led to the drastic enhancement of luminal-amplified CL by zymosan-activated macrophages. As the main component of BN samples is glucose, we compared the effects of all BN samples with that of glucose. It is seen that the effects of all BN preparations were significantly higher that the effect of glucose, but an especially great difference is observed for soluble fractions: thus, solubles BN (Fr. I) and Fr. II enhanced CL produced by zymosan-stimulated macrophages by 2.2-2.8 times in comparison with BN powder and by 3.4 – 4.4 times in comparison with glucose. It follows that the stimulatory activity of all BN preparations cannot be explaned by the presence of glucose and that the activities of soluble BN fractions are significantly higher than the activity of the parent BN powder.
Antioxidant activities of BN preparations were determined in the in vitro experiments.
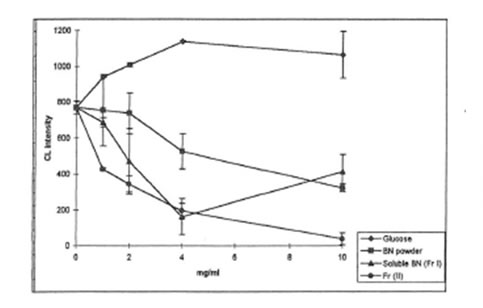
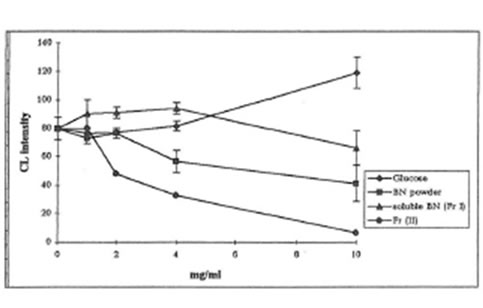
1, 2, 4, or 10 mg/mL of BN samples were added to the incubation mixture containing macrophage suspension (5.0x 105 cells) and luminal (20 µM) or lucigenin (400 µM) in HBSS (pH 7.4, a total volume of 1 mL). The reaction was activated by 0.5 mL (1 mg/mL) opsonized zymosan suspension. As is seen from Figs. 2 and 3, all BN preparations suppressed the formation of active oxygen species measured both luminol- and lucigenin amplified CL in a concentration-dependent manner, while glucose had no effect or even enhanced free radical formation. Antioxidant activity of soluble BN was close to that of BN powder, while Fr. II suppressed the formation of active oxygen species by 10-20 times more efficiently than BN powder or soluble BN (Fr. I).
In vitro antioxidant activities of BN preparations were also measured in the xanthine xanthine oxidase system, which is a well-known classic enzymatic systems widely used for the generation of superoxide ion. The inhibitory effects of BN samples and glucose on lucigenin-amplified CL produced by xanthine oxidase are shown in Table 1. It is seen that all 4 substances inhibited superoxide generation in this enzymatic systems, Soluble BN (Fr. I) and Fr. II being again the most effective scavengers of superoxide ion.
Table 1
The effects of BN preparations and glucose on lucigenin-amplified CL produced by xanhtine-xanthine oxidase system
| Cl intensity | |
| Control | 134±7 |
| Glucose | 108±3 |
| BN powder | 111±7 |
| Soluble | 100±2 |
| Fr. II | 78±1 |
Fig. 1. Lurninol-ampli:fied CL by zymosan-stimulated rat macrophages
(Ex vivo experiments).
Fig. 2. Luminol amplified CL by zymosan stirnulated rat peritoneal macrophages
(In vitro experiments).
.
Fig.3. Lucigenin-amplified CL by zymosan-stimulated rat peritoneal macrophages
(In vitro experiments).
CONCLUSIONS
1. The developed process of the preparation of soluble Bio-normalizer consists of 5 stages. All stages are the up-to-date technological processes, which were reproduced on a pilot plant. Prepared documentation corresponds to the conditions needed for the organization of manufacturing the soluble BN on the factory.
2. Both isolated soluble BN fractions I and II not only retain the immunostimulatory and antioxidant activities of parent BN but exceed it by several times. It may be suggested that the source of this difference is the inhibitory effect of insoluble particles in BN powder.
3. Soluble BN was prepared with a high average yield equal to (73.4±0.6)%. Taking into account that the biological activity of soluble BN exceeds that of BN powder by 1.5-2 times, the developed process allows to receive the final product (soluble BN) with 100-150% yield (in the units of its activity). This fact makes the developed technological process a highly effective one for manufacturing IV BN preparations.
4. On the grounds of biochemical studies, it is concluded that the soluble BN fractions differ from BN powder only by the enhancement of their activities but retain the same biochemical properties. It justifies their application for the treatment of the same illnesses as parent BN.
5. The enhanced biological activities of soluble Fractions I and II are a sound basis for the further investigation of their biochemical and pharmacological properties that will certainly enlarge the medical fields of Bio-normalizer application.