| Title | A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED CLINICAL TRIAL ON THE EFFECT OF BIO-NORMALIZER ON LIVER CIRRHOSIS (FINAL REPORT) |
|---|---|
| Year | 1997 |
| Author | Samar-Sy OM, Espiritu AC, Estadilla-Pornillos EE, Espiritu RS, Corpuz Jr SE, Te HF, Paduga Jr RS, and Osato JA |
| Publisher | Philippine Journal of Internal Medicine |
R9
A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED CLINICAL TRIAL ON THE EFFECT OF BIO-NORMALIZER ON LIVER CIRRHOSIS (FINAL REPORT)
(Submitted to American Journal of Medicine, as of Sept. 1996)
Samar-Sy OM, Espiritu AC, Estadilla-Pornillos EE, Espiritu RS, Corpuz Jr SE, Te HF, Paduga Jr RS, and Osato JA
A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED CLINICAL TRIAL ON THE EFFECT OF BIO-NORMALIZER ON LIVER CIRRHOSIS (FINAL REPORT)
(Submitted to American Journal of Medicine, as of Sept. 1996)
Ofelia Samar-Sy, M.D.1, Andrew Espiritu, M.D.1, Eloisa Estadilla-Pornillos, M.D.1, Rosalinda E spiritu, M.D.1, Silvino Corpuz Jr., M.D.2, Harold Te, M.D.3, Romeo Paduga Jr., M.D.2, and James Akira Osato, PhD.3
1Department of Internal Medicine , Bicol Regional Hospital, Panganiban Ave., Naga City, Philippines.
2Department of Science Technology and Research, Osato Research Foundation, Ayala Tower I, Ayala Ave., Makati City, Philippines
3Sun-O International, Inc. # 12 Minami-machi Bairin, Gifu 500, Japan
ABSTRACT
Bio- Normalizer is a natural health food product processed from papaya with demonstrated antioxidant and immunomodulatory capabilities. A randomized double-blind, placebo-controlled clinical trial was performed at the Bicol Regional Hospital to determine the effect of Bio-Normalizer on patients with liver cirrhosis. Twenty nine patients from different areas of the Bicol Region with confirmed diagnosis of cirrhosis participated of whom 22 were males and 7 were females. Their ages ranged from 20-80 years with the mean of 51.4 years. The patients were randomly assigned to one of two groups, Group A (n=16) was given Bio-Normalizer and Group B (n=13) received placebo for 2 months. The standard medications to treat the complication of cirrhosis were not withheld. The patients were monitored weekly and laboratory examinations were done at 2 weeks interval for 2 months. There were more drop-outs either because of death or withdrawal due to deteriorating condition in Group B. 8 out of the 13 patients who received placebo were not able to finish the 2 months study duration compared to only 3 of the 16 patients in the Bio-Normalizer group. The results showed that 81.2 % of patients survived in Bio-Normalizer group compared to 38.5 % of the patients in the Placebo group. There was statistically significant improvement noted for Bio-Normalizer on the general well-being (x2=6.53, p=0.01) and body malaise scores (x2=4.14, p=0.047). A follow-up study is necessary to increase the number of subjects yield and additional laboratory examinations should be included such as the in vivo detection of the immune system status and the free radical levels.
A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED CLINICAL TRIAL ON THE EFFECT OF BIO-NORMALIZER ON LIVER CIRRHOSIS (FINAL REPORT)
Ofelia Samar-Sy, M.D.1, Andrew Espiritu, M.D.1, Eloisa Estadilla-Pornillos, M.D.1, Rosalinda E spiritu, M.D.1, Silvino Corpuz Jr., M.D.2, Harold Te, M.D.3, Romeo Paduga Jr., M.D.2, and James Akira Osato, PhD.3
INTRODUCTION
Cirrhosis of the liver represents the final common pathway of many types of chronic liver damage characterized by the formation of diffuse fibrosis in the liver and the nodular regeneration of intact hepatic tissues. It is a serious illness with many possible complications and requiring long-term medical supervision and careful management. Current therapeutic measures for the underlying liver pathology are largely for ascites and encelopathy. Overall prognosis for patients with cirrhosis is poor and it is not uncommon cause of death in the adult population.
There is a growing interest in the role of free radicals in many disease processes. Feher, et al.1 have recognized their importance in the pathogenesis and aggravation of tissue damage in chronic liver diseases. Results of studies done by Feher, et al.2 on three hepatoprotective agents indirectly suggest that antioxidant activity might be one of the important factors in the hepatoprotective action of these agents. They found that the low superoxide dismutase (SOD) expression and activity in cirrhotic patients’ lymphocytes and erythrocytes were restored by these drugs. SOD is one of the innate protective free radical scavengers in living system. Lang, et al. 3 In a double-blind clinical trial, demonstrated normalization of elevated levels of SGOT, SGPT, bilirubin in compensated alcoholic cirrhosis patients two free radical scavengers. Feher et al.1 further cited the immunomodulatory effects of free radical scavengers and such immunomodulatory effects may be contributory to the efficacy of the hepatoprotective agents they have tested.
Bio-Normalizer is a natural health food derived from the fermentation of papaya (Carica papaya) and other traditional herbs and yeasts and which is commercially sold in Japan, the Philippines and other countries. The beneficial effects of the products as a health food and even as a therapeutic drug for various simple and serious ailments including liver diseases have been widely attested to by its users although scientific reports or evidences are still deficient to support such claims. Santiago, et al.4 have establish Bio-Normalizer’s scavenging ability on free radicals, most potently on hydroxyl radicals. In vivo studies using Bio-Normalizer have shown that it provides antioxidant protection in the animal models of epilepsy,5 brain-ischemia reperfusion injury,6 and aging.7 Furthermore, Santiago8 reported reduction in previously elevated SGOT and SGPT values in four volunteers after administration of Bio-Normalizer for 2 months. Immunomodulatory effects of this product have also been demonstarated in scientific studies. It was shown to stimulate phagocytes9 and natural killer cell activity10 and increase gamma-interferon in the body.8
By way of a randomized double-blind placebo-controlled clinical trial, this study aimed to evaluate the clinical and laboratory parameters of liver cirrhosis. Results of this study could possibly provide sound scientific support for Bio-Normalizer’s efficacy on cirrhosis of the liver.
METHOLOGY
Patients seen at the Bicol Regional Hospital in Naga City and other nearby centers with impression of liver cirrhosis were screened for possible enrolment in the study. History and physical examination were done on prospective patients. Laboratory procedures including complete blood count, blood chemistry (FBS, BUN, creatinine, electrolytes). Liver function test (protein, bilirubin, transaminases, prothrombin time), hepatitis screening exam, urinalysis, fecalysis with occult blood, chest x-ray, ECG, abdominal ultrasound, and other work-ups deemed necessary were done to screen and/or clear patients for enrolment.
Patients meeting the inclusion criteria were enrolled after providing adequate information about the study and obtaining their written consent. Included in the study were patients with clinical and/or laboratory evidence of liver cirrhosis as evaluated by one of the investigators and confirmed by the research group: 1) presence of sign of chronic liver disease including spider angioma, palmar erythema, gynecomastia in males and testicular atrophy: 2) elevated serum bilirubin and transminases; 3) low total protein and albumin: 4) prolonged prothrombin time: 5) ultrasound findings of a contracted liver with nodular regeneration and 6) for equivocal/doubtful cases, gastroesopageal varices seen on endoscopy. Other patients were screen out when having any one of the criteria set forth: 1) younder than 18 years: 2) residing outside of the Bicol Region: 3) diagnose or with previous history of diabetes mellitus: 4) with renal diseases with albuminuria of ++ or higher and elevated levels of BUN (>20 mg.dL) and creatinine (>1.2 mg.dL): 5) with history of congestive heart failure secondary to valvular, hypertensive, or ischemic heart disease: 6) with chronic obstructive pulmonary disease (COPD) with or without cor pulmunale: 7) have previously taken Bionormalizer
During 12 months of the study, 29 patients have been included. The subject were classified into three classes according to Child’s criteria of liver fuctional reserve (Child, CG 111 and turcotte, J; In: The Liver and Portal Hypertension. Child, CG 111 (ed.). Saunders, 1995). Each patients was randomly assigned to one of two groups via computer generated table of random numbers. Both groups received the standards regimen of the attending physician. The test substance (Bionormalizer) placebo served as an adjunct to the standard regimen. The standard recommended dosaging procedure for Bio-Normalizer was followed which was to start each patient on 1 sachet (3 grams) of the test substance per orem at bedtime and the patient monitored weekly for subjective improvement/s. The maximum dose was set at 6 sachet/night. Each patient continued to receive the determined dose or number of sachets nightly to complete 60 days or 2 months. . Group A patients were given Bio-Normalizer while Group B patients received placebo.
Baseline measurements of the clinical and laboratory parameters were taken for each subject. Clinical parameters included a) presence of jaundice, edema, anorexia and body malaise; b) weight; c) abdominal girth at the level of the umbilicus; and d) general well-being score and these were monitored weekly thereafter. Laboratory parameters, on the other hand, were taken every 2 weeks for 2 months and these were a) complete blood count and b) liver function tests (DB, IB, SGOT, SGPT, prothrombin time, total protein and albumin).
Differences in survival rates between the Placebo and Bio-Normalizer groups were tested using the proportional hazards model with Child’s class categorization of patients as covariate. For the clinical parameters, the status of the patients at the start of the study and at the end were compared and response to treatment were categorized as positive or negative. Differences between the two groups were compared using X2-test of homogeneity. In the case of laboratory parameters, the percentage relative change between the baseline and endline values were computed per patient and the mean change between the Placebo and Bio-Normalizer groups were compared using the t-test. The computer software’s used for data processing and analysis were EPIINFO 6 and EGRET.
3. RESULTS
3.1 Profile of Subjects
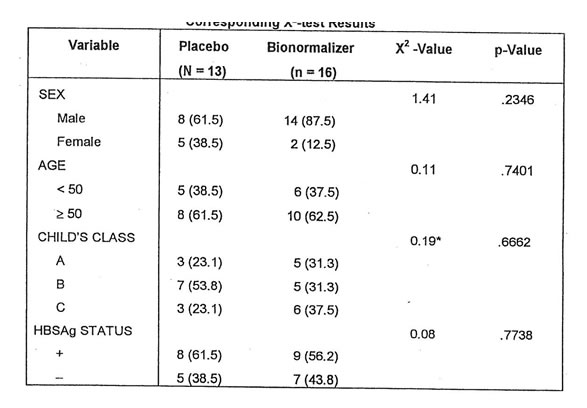
The study included 29 patients with alcoholic cirrhosis of which, 13 were randomly assigned to the placebo group and the other 16 to the Bionormalizer group. There were more male than females patients for both groups. Their ages range from 35-80 for the placebo group with a mean of 53.5 years. For the Bionormalizer group, their age range from 20 to 66, with a mean of 49.3 years. When the patients were classified according to the different categories of Child’s Class, the Bionormalizer group were equally divided among the three categories. On the other hand, a little over half (53.8%) of the Placebo group were categorized under Class B. The proportion of subjects who were positive for HBSAg were about the same for both groups. To determine whether the profile of subjects in the Placebo and Bio-Normalizer groups were comparable with respect to each of these variables, the X2-test was applied. The results showed no significant differences between the two groups for each of these variables. These are shown in Table I.
Table I. Distribution of Subjects according to Selected Variables and Corresponding X2-test Results.
* Computed by lumping together classes A & B, to meet sample size requirements for the validity of the X-test.
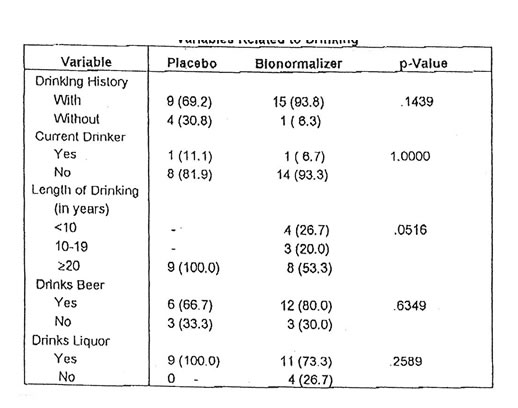
Since alcohol is an etiologic factor for cirrhosis, the drinking history of the subjects were also ascertained. The results show that almost everyone (93.8%) from the Bio-Normalizer group has a history of drinking while the corresponding magnitude for the placebo group is also high (69.2%). There was only 1 current drinker for both groups at the time of the study. The length of time the subjects have been drinking ranged from 1 to 48 years, with a mean of 30.2 years for those in the placebo group and 23.7 years for those in the Bio-Normalizer group. Two out of every 3 subjects (66.7%) with a drinking history in the placebo group were beer drinkers while the corresponding proportion in the Bio-Normalizer group is slightly higher (80.0%). Everyone in the placebo group drank liquor while the corresponding proportion in the Bio-Normalizer group was lower at 73.3%. The Fisher’s exact test done to determine the comparability of the two groups with respect to different aspects of the drinking profile of the subjects showed no significant differences between them. These are shown in Table II.
Table II. Distribution of Subjects According to selected Variables Related to Drinking
*The p-values are derived using Fisher’s exact probability test due to very limited sample sizes. The p value corresponding to length of drinking is based on the X2-test homogeneity.
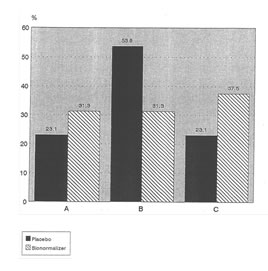
Through the 8 weeks observation period of each patient, 11 patients from both groups were not able to complete the 2 months either because of death or decoding and subsequent removal from the study due to worsening condition. Three patients died among 16 in Bio-Normalizer for an 18.75% mortality rate within two months and all belong to Child’s class C. In the placebo group, 6 patients died among 13 (46.15% mortality rate). Two among the latter group were withdrawn from the study due worsening condition. The 6 patients had different Child’s classifications (see Table II). A comparison of the profile of patients in the Placebo and Bio-Normalizer groups according to Child’s class is shown in Figure 1.
Clinical Parameters
Among the indicators used to assess the effect of Bio-Normalizer were clinical parameters namely the presence of edema, jaundice, anorexia, malaise, bleeding and general well-being. Patients were monitored according to each of these parameters every two weeks and their status were noted according to the degree of severity or frequency of occurrence of the condition, as the case may be. In the case of the assessment of general well-being for example, patients were given a 5-point score ranging from 0 which indicates an excellent condition to a score of 4 indicating a very poor status. To facilitate data analysis, the status of the patients between baseline and the endline of the study were compared and categorized according to any 4 categories namely: good throughout, improved, unchanged and deteriorated. The first 2 categories were considered as positive responses and the latter 2 categories as negative responses. The profile of patients according to these responses were compared between the Placebo and Bio-Normalizer groups using the X2-test of homogeneity.
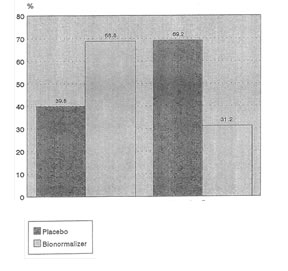
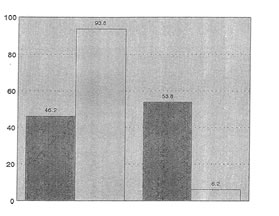
The results of the statistical test show that among the clinical parameters, significant differences were observed only for body malaise (X2=4.14,p=0.047) and general well-being (X2=6.53, p=+0.01). For both parameters, the proportion of patients with positive responses were about 2x higher in the Bio-Normalizer group compared to the Placebo group. These are shown in Figure 2 and 3, respectively.
In addition to the above clinical parameters, the weight and abdominal girth of patients were also monitored and compared between the two groups. The percent relative change in these parameters between the baseline and endline values were both lower for the Bio-Normalizer compared to the Placebo group. The differences however were not statistically significant.
Laboratory Parameters
The effect of Bio-Normalizer on laboratory parameters were likewise monitored. Their actual values were recorded every 2 weeks for 2 months and the percent relative-change in the baseline and endline value of each patient was computed. The results shown that most laboratory parameters, the baseline values were higher compared to the endline values resulting in a negative % relative-change. In addition, the % relative-change were generally smaller in the Bio-Normalizer group compared to the Placebo group. Such differences however were not statistically significant.
Survival of Patients
The most important indicator used to assess the effectiveness of Bio-Normalizer was the survival of patients. The result showed large differences between the 2 groups with respect to this variable. Among those in the Placebo group, only 38.5% of the patients survived compared to 81.2% in the Bio-Normalizer group. These are shown in Table III. All the 3 patients who died in the Bio-Normalizer group were due to cardio-pulmonary arrest secondary to multiple organ failure secondary to cirrhosis. In the case of the Placebo group, 3 of the deaths had the same cause as that of the deaths in the Bio-Normalizer group while the other 3 deaths were due to massive bleeding due to secondary esophageal varices secondary to cirrhosis. Two of the patients in the Placebo group had to be withdrawn due to worsening condition.
Table III. Distribution of Patients according to Group and Status at the end of the Study
| Status | Placebo | Bio-Nomalizer | Total |
| Suvived | 5 (38.5) | 13 (81.2) | 18 (62.1) |
| Withdrawn | 2 (15.4) | 0 (0) | 2 (6.9) |
| Died | 6 (46.2) | 3 (18.8) | 9 (31.0) |
| Total | 13 (44.8) | 16 (55.2( | 29 (100.0) |
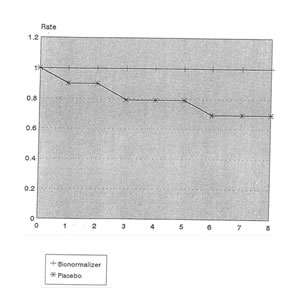
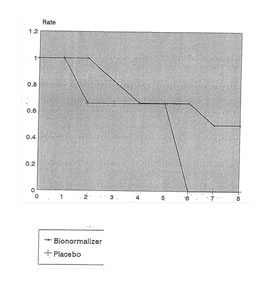
To compare the survival rate of patients in both groups, the proportional hazards model was used. Since the 2 groups were not very comparable with respect to the severity of the cases, this child’s class categorization of patients was used as a covariate in the statistical model. For this purpose, Child’s class A and B were lumped together as one category due to small sample sizes while Child’s class C was maintained as a separate group. The results showed a significant difference between the survival rates of the Placebo and Bio-Normalizer groups (Livelihood Ratio Stat.=7.76, p=0.005). The computed hazard ratio was 0.2243% implying that the probability of dying among those in the Bio-Normalizer group is only 22.43% compared to those in the Placebo group, controlling for the severity of the disease. Figures 4 and 5 shows the comparison of the survival rates for the Placebo and Bio-Normalizer groups for Class A/B and C patients.
DISCUSSION
The study showed general improvement in the clinical signs and symptoms such as jaundice, edema, anorexia, malaise and general well-being although statistically significant finding was only noted on body malaise and general well-being. There is a trend toward improvement of the laboratory parameters such as bilirubin, SGPT, total protein, albumin, and prothrombin time but differences were not statistically significant. There is also a decrease of abdominal girth with corresponding decrease in weight as ascites diminished although these cannot be attributed to Bionormalizer alone because the patients also took diuretic drug (Spironolactone) More important findings were the statistically significant difference between the survival rates of the Placebo and Bionormalizer groups. The computed hazard ratio showed less probability of dying in the Bio-Normalizer group compared to the Placebo group.
Bio-Normalizer intake is well tolerated by the patients with no side effects seen. Some patients even requested additional supplies of Bio-Normalizer at the end of the study because according to them, Bio-Normalizer increased their appetite, improved their gastro-intestinal and urinary output, improved their sleeping pattern and vitality as evidently shown in the improvement of genera well-being. So Bio-Normalizer therapy was extended to these patients for another month.
Bio-Normalizer has been shown to enhance the activities of the immune system. Specifically, it has been found to stimulate the production of gamma-interferon (8), one of the substances produced by the immune cells in response to viruses. It has a general function of regulating the activities of the immune system. Interferon activates the macrophage, natural killer cells and lymphocytes to fight infections. In addition, Bio-Normalizer has been found to enhance the activity of Natural Killer cells directly.(10) The latter is thought to be involved in the destruction of virus infected cells, tumor cells anf foreign bodies. Bio-Normalizer also has the capacity to inhibit the growth of gram (-) bacteria like Escherichia coli (11) which is responsible for endotoxin formation, believed to be increased in cases of liver cirrhosis.
According to Shiratori et al., (12) patients with liver cirrhosis had significantly increased superoxide anion generating capacity of polymorphonuclear cell as measured by chemiluminescence in the presence of luminol. Bio-Normalizer is a free radical scavenger as proved by previous studies scavenging hydroxyl as well as superoxide radicals.
These immunomodulatory and free radical regulating actions of Bio-Normalizer might be partly responsible for the improvements noted in the patients.
CONCLUSION
Base on the result of the study and our firsthand experience in the improvement of the patients, Bio-Normalizer may have a in the management of patients with liver cirrhosis as it improved their quality of life and prolong the survival rate.
A follow-up studies is necessary with multicenter approach to increase the number of subjects. Other necessary laboratory examinations should be included such as the in vivo detection on the immune system status and the free radical and antioxidant levels. Follow up studies of patients should be extended to a longer period of time for survival analysis.
REFERENCES
- Feher J, Lang I, Deak G et al: “Free Radicals in Tissue Damage in Liver Diseases and Therapeutic Approach.”
Tokai Journal of Experimental and Clinical Medicine (11 Suppl): 121, 1990. - Feher J, Lang I, Nekam K et al: “Effects of Free Radicals on Superoxide Dismutase (SOD) Enzyme in patients with Alcoholic Cirrhosis.” Acta Medica Hungarica 1996:45(3-4):265, 1996.
- Lang I, Nekam K, Deak G et al: “Immunomodulatory and Hepatoprotective Effects of in Vivo Treatment with Free Radical Scavengers.” Italian Journal of Gastroenterology 22(5):283, 1990
- Santiago L, Osato J, Hiramatsu M et al: “Free Radical Scavenging Action of Bio-catalyzer a. r. No. 11 (Bio-Normalizer) and its by Product.” Free Radical Biol. Med. 11:379, 1991
- Santiago L, Osato J, Kabuto h, Mori A: “Decreased Release of Monoamine Metabolites in Iron-induced Epileptogenic Focus in in the Rat Following Administration of Bio-catalyzer.” Med. Sci. Res. 21(4):39, 1993
- Santiago L, Osato J, Ogawa N, Mori A: “Antioxidant Protection of Bio-Normalizer In Cerebral Ischemia-reperfusion Injury in the Gerbil.” Neuro Report 4(8):1031, 1993
- Santiago L, Osato J, Liu J, Mori A: “ Age-related Increases in Superoxide Dismutase Activity and Thiobarbituric Acid-reactive Substances: Effect of Bio-Catalyzer in Rat Brain.” Neurochemical Research 18(6):711, 1993.
- Santiago L, Uno K, Osato J et al: “Effects of Bio-Normalizer on Serum Components and Immunological Function in Humans.” Neurosciences 20(Suppl): 149, 1994
- Osato J, Afanas’ev I, Korkina E et al: “BN as Modulator on Phagocytosis and Free Radical Production by Murine Inflamed Neutrophils and Macrophages.” Physical Clinical Biology & Medicine Vol 2(2):1, 1995.
- Okuda H, Ohminami H, Zhou A et al: “Studies on Biological Activities on Bio-Normalizer.” The Clinical report 27(11):4249, 1993.
- Sherlock S, Dooley J: Diseases of the Liver and Gastrointestinal Tracts, 9th Ed. Australia: Blackwell Scientific Publication, 1993, p. 160.
- Osato J, Bernas G, Santiago L et al: “Antimicrobial Potential of Bio-Normalizer against Enteric Microorganisms.” Acts Manilana 42,9, 1994.
- Shinatori Y, Kawase T, Sugimoto T:”Superoxide Anion Generating Capacity of Polymorphonuclear Cells in Patients with Liver Cirrhosis.” Gastroenterogica Japonica 21(1):30, 1986.
Fig 1 Percentage of Subjects According to Group and Child’s Classification
Child’s Class
Fig. 2 Comparison of Placebo and Bio-Normalizer Groups
According to response for Body Melaise
Child’s Class
Fig.3 Comparison of Placebo and BioNormalizer Groups
According to Response to General Well-Being
Child’s Class
Fig.4 Survival rates of Child’s Class A and B Patients With Cirrhosis of the Liver According to Treatment Group
Time (in weeks)
Child’s Class
Fig. 5 Survival Rates of Child’s Class C Patients with Cirrhosis of the Liver According to Treatment Group
Time (in weeks)