| Title | NATURE’S FOOD ANTIOXIDANTS IN HEALTH AND DISEASE PREVENTION |
|---|---|
| Year | 1995 |
| Author | Librado A. SANTIAGO, James Akira OSATO, and Akitane MORI a |
| Publisher | Magnetic Resonance in Medicine |
Nature’s Food Antioxidants in Health and Disease Prevention
Librado A. SANTIAGOa,b, James Akira OSATOb,c and Akitane MORI a
aDepartment of Neuroscience, Okayama University Medical School, Okayama 700, Japan, bOsato Research Institute, Gifu 500, Japan and cThe United Graduate School of Agricultural Science, Gifu University, Gifu 500, Japan
A trend for all things natural is now seen in the growing public inclination to turn back to the basics and in the increasing applications of natural preventive medicines, generating much of the research interests on the antioxidant properties of such products and the benefits in health, nutrition, and disease prevention. By seeing disease as a disturbance within the patient’s body system, changes in the normal redox equilibrium, i.e., excess production of reactive oxygen species such as hydroxyl, superoxide, hydrogen peroxide and other radicals, can cause damage to DNA, proteins, lipids, among others and result to various illnesses and in extreme cases, death. The body is highly endowed with enzymatic and non-enzymatic antioxidant defenses, which however, are depleted when the tissue is continuously attacked and damaged by free radicals. Supplementation with food dietary antioxidants is thus regarded as an important yet conservative approach in natural remedies against free radical-induced diseases. Natural medicines and supplements derived from numerous plant materials and microorganisms abound but their antioxidant activity is not fully understood. Our work has focused on the electron spin resonance (ESR) spectroscopic detection of the free radical scavenging activity of some of these food antioxidants such as papaya, baker’s yeast, soybean paste (miso), rice bran, horseradish (wasabi), and Bio-normalizer.
Materials and Methods
All reagents and chemicals used were of the highest grade available from commercial suppliers. A 2~3 week old Philippine variety unripe papaya; a yellow variety miso from Kaneko-Miso Ltd., Tokushima; baker’s yeast Saccharomyces cerevisiae under the trademark Saf-Instant (S.I. Lesaffre, France); wasabi from S&B Co., Tokyo; rice bran from commercial supplier; and Bio-catalyzer a.r No.11 (Bio-normalizer) from Sun-O International Inc., Gifu were used. The cerebral cortex of male adult Sprague-Dawley rats (Clea Japan Inc., Tokyo) was dissected and homogenized (0.05% w/v) in 0.1 M Tris-HCl buffer, pH 7.4. Test food samples were homogenized then centrifuged at 1000 rpm for 5 min at 4ºC. By ESR spectrometry/spin trapping (JEOL, JES-FE1XG, Tokyo), the reactions of these water-soluble extracts with the different free radicals generated in organic, aqueous, and brain homogenate systems such as 1-1 diphenyl-2-picrylhydrazyl (DPPH), hydroxyl, superoxide, and carbon-centered were tested as stated in previous reports (1~4). The superoxide dismutate (SOD) activity, assayed using ESR (2,9) was based on the ability of 25 mg/ml samples to dismutase almost completely the superoxide radicals generated from the hypoxanthine-xanthine oxidase system.
Results and Discussion
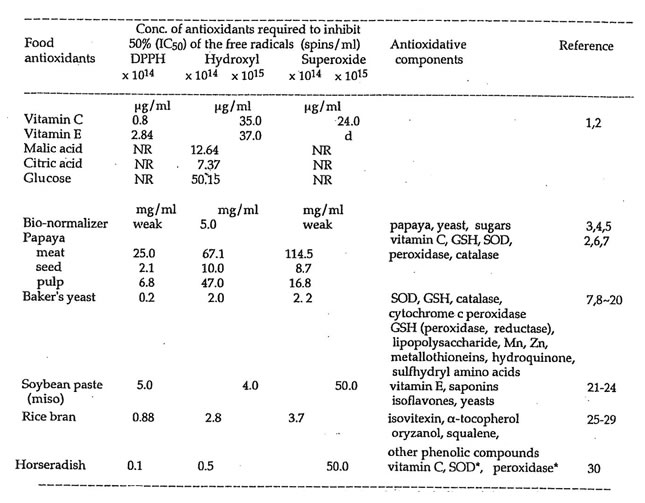
Table 1 shows the IC50 of the food antioxidants and their corresponding antioxidative constituents required to inhibit the DPPH, hydroxyl and superoxide radicals. These crude/unpurified food antioxidants are thousand-fold less potent than their pure specific components such as vitamins C and E, malic and citric acids, and glucose. Except for papaya and miso, all samples were analyzed on dry weight basis such that the differences in the moisture content may have partly contributed to differences on the antioxidant potency of these food antioxidants. 200 mg/ml baker’s yeast scavenged 100% of the hydrogen radicals (7.8 x 1014 spins/ml) and 94.8% of the carbon-centered radicals (3.4 x 1016 spins/ml). Similarly, it required 180 mg/ml miso to quench 90% and 82% of the hydrogen radicals (1.7 x 1013 spins/ml) and carbon-centered radicals (3.9 x 1013 spins/ml), respectively. 25 mg/ml rice bran inhibited 60.9% of carbon-centered radicals (6.6 x 1012 spins/ml). Because the bran is insoluble higher concentrations could not be prepared and tested. The papaya meat, seed and pulp contains 32, 98 and 33 units/ml of SOD-like activity while miso, rice bran, and baker’s yeast have 58, 65, and 75 units/ml, respectively. With both their intrinsic nutrient values and antioxidative protection, these nature’s food antioxidants are envisaged as double-pronged approaches in nutrition, health and medicine.
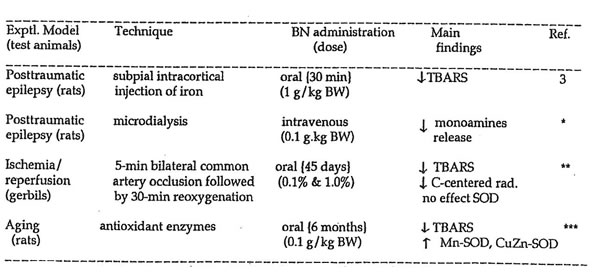
Of these dietary food antioxidants, Bio-normalizer – a fermented health food from papaya, yeast and other traditional Japanese foodstuffs – was studied in detail using experimental animal subjected to oxidative stress as in the models of post traumatic epilepsy, intracerebral microdialysis, ischemia/reperfusion injury, and aging, As shown in Table 2, Bio-normalizer exerted antioxidant protection in all of these animal models, thereby supporting its purported preventive and therapeutic actions.
Table 1. ESR spectroscopic detection of the free radical scavenging action of the water-soluble extracts of some naturally occurring food antioxidants.
NR – no reaction; d – delayed quenching action observed after 6-9 min in lieu of the usual analytical time of 50s; * commercially available; GSH – glutathione; SOD – superoxide dismutase.
Table 2. Antioxidant effects of Bio-normalizer on different experimental amal models.
TBARS, thiobarbituric acid reactive substances; *Med. Sci. Res., 1992; 21: 139-141, **NeuroReport, 1993; 4: 1031-1034, ***Neurochem. Res., 1993, 18: 711-717.
References
- Santiago LA, Hiramatsu M and Mori A. 1992. J. Nutr. Sci. Vitaminol., 38: 297-304.
- Osato JA, Santiago LA, Remo G et al. 1993. Life Sci., 53: 1383-1389.
- Santiago LA, Osato JA, Hiramatsu etal. 1991. Free Radical Biol. Med., 11: 379-383.
- Santiago LA, Osato JA and Mori A. 1992. Med. Sci. Res., 20: 27-28.
- Osato JA, Cuadra MS, Santiago LA et al. Proc. Int. Conference on Bioradicals Detected by ESR spectroscopy (in press).
- Webman EJ, Edlin G and Mower HF. 1989. Int. J. Radiat. Biol. 55: 347-351.
- Halliwell B and Gutteridge GMC. 1989. Free Radicals in Biology & Medicine. Clarendon Press: Oxford.
- Santiago LA, Hiramatsu M and Mori A. 1991. Med. Sci. Res. 19: 867-868.
- Santiago LA and Mori A. 1993. Arch. Biochem. Biophys. 306: 16-21.
- Elsken MT, Jaspers CJ and Penninckx MX. 1991. J. Gen. Microbiol. 137: 637-644.
- Kohama Y, Teramoto T, Kayamori Y et al. 1990. Agric. Biol. Chem. 54: 3051-3052.
- Westerbeek-Marres CAM, Moore MM and Author AP. 1988. Eur. J. Biochem., 174: 611-620.
- Galazzo F, Ciriolo MR, Carri MT et al. 1991. Eur. J. Biochem., 196: 545-549.
- Ravindranath SD and Fridovich I. 1975. J. Biol. Chem., 250: 6107-6112.
- Chang EC and Kosman DJ. 1989. J. Biol. Chem., 264: 12172-12178.
- van Loon APGM, Pesold-Hurt B and Schatz G. 1986. Proc Natl. Acad. Sci. USA, 83: 3820-3824.
- Bermingham-McDonogh O, Gralla FB and Valentine JS. 1988. Proc. Natl. Acad. Sci. USA, 85: 4789-4793.
- Zimniak P, Hartter E, Woloszczuk W and Ruis H. 1976. J. Biochem., 71: 393-398.
- Arscott LD, Thorpe C and William CH, Jr. 1981. Biochemistry, 20: 1513-1520.
- Nelson CE, Stizman EV. Kang CH et al. 1977. Anal. Biochem., 83: 622-631.
- Naim M, Gestetner B, Bondi A et al. 1976. J. Agric. Food. Chem. 24: 1174-1177.
- Chow CK and Drapper HH. 1974. Int. J. Vitaminol. Nutr. Res. 44: 396-403.
- Naim M, Gestetner B, Kirson I et al. 1974. Phytochemistry, 12: 169-170.
- Ohminami H, Kimura Y, Okuda H et al. 1984. Planta Med., 50: 440-441.
- Changbumrung S, Buavatana T and Migasena P. 1980. Int. J. Vitamin. Nutr. Res., 50: 242-246.
- Tajima K, Sakamoto M, Okada K., 1983 Biochem. Biophys. Res. Comm., 115: 1002-1008.
- Ramarathnam N, Osawa T, Namiki M et al. 1986. J. Sci. Food Agric. 37: 719-726.
- Ramarathnam N, Osawa T, Namiki M et al. 1989. J. Agric. Food Chem., 37: 316-319.
- Windholz M and Budavari S. (eds.) 1976. The Merck Index, Merck & Co. Inc., USA, pp. 8547-8548.
- Santiago LA, Hiramatsu M and Mori A. 1992. In: Yagi K, Kondo T, Niki E et al. (eds.), Oxygen Radicals, Elsevier Science Publisheres, pp 819-822.
Key words: Free radical scavengers, food antioxidants, ESR spectroscopy