| Title | IMPROVEMENT OF HEMORHEOLOGICAL ABNORMALITIES IN ALCOHOLICS BY AN ORAL ANTIOXIDANT |
|---|---|
| Year | |
| Author | Prof. Francesco Marotta |
| Publisher |
Improvement of Hemorheological Abnormalities in Alcoholics by an Oral Antioxidant
F Marotta, P Safran1, H Tajiri2, G Princess3, H Anzulovic3, GM Idéo, A Rouge2, MG Seal1, G ldéo
Hepatogastroenterology Dept.,S. Giuseppe Hospital, Milano, Italy; 1SFJO & Labs., Paris, France; 2Research Division, Shikoku Cancer Center Hospital, Matsuyama, Japan; 3α-Ω Technolab, Geneva, Switzerland
Corresponding Author: Prof. Francesco Marotta, MD, PhD, via Pisanello 4, 20146 Milano, Italy
E-mail: fmarchimede@libero.it
ABSTRACT
Background/Aims: It has been shown that alcohol impairs erythrocyte (red blood cell) membrane fluidity and lipid composition. The aim of this study was to test the effect of a novel acid-resistant antioxidant on the hemorrheology in alcoholics.
Methodology: Thirty alcoholics (25 males, 5 females; mean age: 42 years; range: 31-54; 150g ethanol/day for 3-5 years) were enrolled into the study. Patients were randomly and double-blindly allocated in to 2 groups which were given, for a 2 week period, 18g/day of Bionormalizer (obtained from biofermentation of carica Papaya, pennisetum purpureum, sechium edule, Osato Res. Foundation, Gifu, Japan) dissolved in 5mL of water at bedtime and 3 hours prior to examination. Placebo consisted of flavored sugar. Healthy teetotalers served as control. On the examination day, blood samples were taken for testing: routine tests, plasma glutathione, ascorbic acid, selenium, plasma lipid hydroperoxides and α-tocopherol. Erythrocytes were separated and tested for red blood cell malonyldialdehyde and glutathione content. The hemorheological studies were follows: blood and plasma viscosity, whole blood filterability, red blood cell membrane fluidity by electron spin resonance, red blood cell aggregation index by photometric rheoscopy and red blood cell deformability by ektacytometry.
Results: As compared to healthy controls, alcoholics on placebo treatment showed no change of plasma viscosity but a significantly higher red blood cell malonyldialdehyde, blood viscosity (P<0.05) and lower plasma glutathione, whole blood filterability and red blood cell fluidity (P<0.01). No relationship appeared between biochemical tests and red blood cell membrane fluidity. Bionormalizer group showed a significant recovery, to control values of either blood viscosity and whole blood filterabitity (P<0.01) and a partial, although significant, improvement of red blood cell membrane fluidity, red blood cell malonyldialdehyde and plasma glutathione (P<0.05). As compared to healthy control, red blood cell aggregation decreased in alcoholics (P<0.05) and was not affected by Bionormalizer. However, Bionormalizer significantly improved the reduced red blood cell deformability (P<0.05 vs. alcoholics) and this parameter correlated with red blood cell malonyldialdehyde (r: 0.62, P< 0.05).
Conclusions: These preliminary data suggest that an effective antioxidant supplementation is able to improve the hemorrheology in alcoholics either by directly affecting the ethanol-related lipoperoxidation and xanthine oxidase system activation and/or by modifying red blood cell membrane characteristics.
KEY WORDS: Hemorrheology; Erythrocytes; Natural oral antioxidant; Lipoperoxidation
ABBREVIATIONS: Red Blood Cell (RBC); Bionormalizer (BN); Glutathione (GSH); Malonyldialdehyde (MDA); High-Performance Liquid Chromatography (HPLC); Packed- Cell Volume (PCV); Elongation Indexes (EI); Pascal (Pa); Aspartate Aminotransferase (AST); Alanine Aminotransferase (ALT); ᵧ-Glutamytransferase (ᵧGT).
Introduction
It has been shown in the past that alcohol might impair erythrocyte membrane fluidity and lipid composition (1,2). Indeed, circulating erythrocyts are exposed to high oxygen tension and they also abound in iron which is a transitional metal promoting the formation of oxygen free radicals. In particular, low molecular thiol concentration has been pointed out as a main pathophysiological step of such derangement (3). On clinical grounds, there have been some scantly in vivo and in vitro reports on the beneficial effect of glutathione (GSH) on the hemorhelogical abnormalities associated with atherosclerosis (4). Consequently, in alcoholics in whom free radical-related phenomena are widely involved, a role of GSH, which alcoholic patients may greatly lack in their blood and liver tissue (5-7), is likely to take place in this issue although no specific dietary supplementation trial has been tested as yet. We have recently shown that a novel acid-resistant antioxidant, which results from yeast-biofermentation of known tropical plants, i.e .,Bionormalizer, is able to exert a rapid and potent protective action on ethanol-induced gastric mucosal damage (8). Therefore the purpose of the present investigation was to study the hemorheological parameters in a group of alcoholics without overt liver failure or ongoing medications while testing the effect of the above natural product endowed with atioxidative properties.
METHODOLOGY
Thirty alcoholics without overt liver disease (25 males, 5 females; mean age: 42 years; range: 31-54, 150g ethanol/day for 3-5years) were interviewed with special attention to their ethanol consumption, drinking behavior and dietary-vitamin intake by a standardized food-composition table. The most used source of alcohol was wine, which was consumed during and between meals. There was no history of viral hepatitis, dislipidemia or vascular disease nor evidence of ongoing hematological disturbance. Patients were randomly and double-blindly allocated into 2 groups, previously matched for dietary and alcohol intake, and were given 18g/day of Bionormalizer (BN, obtained from biofermentation of carica papaya, pennisetum purpureum, sechium edule, Osato Research Foundation, Gifu, Japan) (Table 1) dissolved in 5mL of water at bedtime and 3 hours prior to examination for a period of 2 weeks. Placebo consisted of flavored sugar, which was devoid of any antioxidant property. Control subjects consisted of 10 healthy teetotaler subjects matched for age and gender with patients’ population. On the examination day, blood samples were taken for testing: routine tests, acetaldehyde by head space gas chromatography (9) GSH (10). For assessing the variation of the blood antioxidant status the following parameters were measured: Plasma lipid hydroperoxides following the method of Ohishi et al. (11) by hemoglobin catalyzed oxidation of 10–N–methyIcarmoyl–3, 7-dimethylamino-10-H-phenothiazione after treatment with phospholipase D. Accordingly, cumene hydroperoxide was used as the related standard. Plasma content of α-tocopherol was measured by high-performance liquid chromatography (HPLC) analysis (values read by fluorescent detector at 296nm excitation and 325nm emission wavelength, respectively), as described by Burton et al. (12). Part of the plasma sample was separated by centrifugation at 4°C and the leukocyte layer was promptly removed by aspiration. The remaining erythrocytes were washed in 5mL ice-cold phosphate buffered saline with a pH of 7.4 and sedimented for 5min at 1500rpm. Aliquots of 0.5mL of the packed erythrocytes were added to 1mL of 50% trichloroacetic acid. For assessing the erythrocyte (RBC)-malonyldialdehyde (MDA), HPLC analysis was used according to Esterbauer et al. (13). Briefly, 0.2mL of packed erythrocytes were thoroughly mixed with the same volume of ice-cold acetonitrile in 1.5-mL Eppendorf tubes. Following 20min of extraction in ice, the samples were centrifuged for 1min at 13.000rpm and 20μL of supernatant was added to the HPLC system (Lichrospher column, Merck, Darmstadt, Germany) with acetonitrile/30mM TRIS buffer, pH 7.4 (1:9 vol/vol) as the eluent. The effluent was monitored at a 270-nm wavelength and the MDA peak in the chromatogram was identified by comparison with that of free MDA standard, which was freshly prepared by hydrolysis of MDA-bisdiacetal in 1% sulphuric acid solution. Glutathione (GSH) content of erythrocytes was measured by spectrophotometry at 412nm by the reaction with dithionitrobenzoic acid (14).
Hemorheological Studies
Blood and plasma viscosity was measured by a Wells-Brookfield cone/plate viscosimeter operated at 225 sec-1. Obtained values were corrected by hematocrit levels, by setting the readings at a standard (45%) reference. Whole blood filterability was assessed following a slightly modified method of Reid et al. (15). Briefly,6-mL aliquots of blood samples were immediately placed in test tubes with 0.3mL of 2% EDTA as anticoagulant (in 0.1M phosphate buffer at pH 7.4). Then, blood specimens were thermostatically maintained at 37°C. Within the following 10min,1 mL of whole blood was filtered through a 5μm-pore nucleopore membrane at a negative pressure of 20cm of water. Filtration time was measured starting from the time blood initially passed through the membrane until the whole 1mL of blood was finally collected. The mean value of three separate measurements was corrected using the formula 60/FT x PCV/100, PCV representing the packed-cell volume and expressed volume of blood filtered per min. For RBC-membrane fluidity determination,8µg of spin labels 5-DS and 16-DS per mg protein were dissolved then evaporated under nitrogen. One hundred microliters of erythrocytes suspension was added to it and the sample was mixed for 2min in a vortex mixer. Thereafter, the sample was placed in a capillary tube and the spin labels were assayed using an electron spin resonance spectrometer (JEOL JES-FE, Tokyo, Japan). The spectrometer analysis system was set as follows: magnetic
|
TABLE 1 Chemical Analysis of Bionormalizer (100g) |
| Available carbohydrate, g 90.7 |
| Moisture, g 8.9 |
| Protein, g 0.3 |
| Fat, g none |
| Ash, g 0.1 |
| Folic acid, μg 2 |
| Niacin, mg 0.24 |
| Amino acids, mg |
| Arginine 16 |
| Lysine 6 |
| Histidine 5 |
| Phenylalanine 11 |
| Tyrosine 9 |
| Leucine 18 |
| Isoleucine 9 |
| Methionine 5 |
| Valine 13 |
| Alanine 12 |
| Glycine 11 |
| Proline 37 |
| Glutamic acid 11 |
| Serine 11 |
| Threonine 8 |
| Aspartic acid 27 |
| Tryptophan 2 |
Carotene,cyanocobalamin, thiamin, ribonavin, ascorbic acid, tocopherols and selenium: not detected.
Japan Food Research Laboratories: Report No
397100396-007, Tokyo, December 16, 1997.
field, 322±5mT and 10mT; modulation, 0.2mT; response, 0.3 sec; sweep time,2 and 4min; amplitude,3.2 x1.000 and temperature 37°C. The polarity-correlation order parameter was calculated from the spectra as described by Hubbel and McConnell (16) in the 5-DS-label fraction. The 16-DS-label sample was used to measure the motion parameter, which relates to the rotational correlation time in the hydrophobic region, according to the method of Elect and Imesi (17). For RBC-aggregation assessment a photometry rheoscope interfaced to a digital computer was used. RBC aggregation was evaluated, after as hearing period at 600 sec-1, at stasis (M) and at a low shear rate of 3 sec-1 (Ml) by integrating the light transmission through the sample for 10 sec as described by Bauersachs et al (18). Disaggregation shear rate (γTmin) was measured by shearing RBC suspensions at 11 separated levels of shear rate between 7.5 and 500 sec-1 then fitting a third order polynomial through the computer in order to calculate the shear rate at minimal light transmission. Thus, an increase of M (aggregation at stasis), M1 (aggregation promoted by low shear) or γTmin (minimal shear rate able to fully disperse RBC aggregates) represents an enhanced RBC aggregation. RBC aggregation was measured in autologous plasma and in 3% dextran 70. All measurements were performed at 45% hematocrit and at room temperature (22±1°C). RBC deformability was quantified at various fluid shear stresses by laser diffraction analysis, by using an ektacytometer, as described by Hardeman et al. (19). Briefly, the blood sample was sheared in a glass made Couette system with a gap of 0.3mm between the cylinders. The diffraction pattern was analyzed by a microcomputer and elongation indexes (EI) were calculated for shear rates between 0.5-50 Pascal (Pa), where an increase of EI indicates greater RBC deformability. RBC were suspended in a PBS dextran 70 solution (the system was set as follows. Viscosity: 24.8mPa; osmolarity: 293mOsm/Kg at a cell count of 2×107mL; temperature: 25°C).
Statistics
Results are expressed as mean ± standard error (S.E). Statistical comparisons between groups were done by “paired t test” and P values smaller than 0.05 were accepted statistically significant. Correlations were tested using the Kendall-Tau test for nonparametric data.
RESULTS
When considering the non-alcohol energy intake, there was no significant difference between alcoholics and controls as far as their dietary habit was concerned.
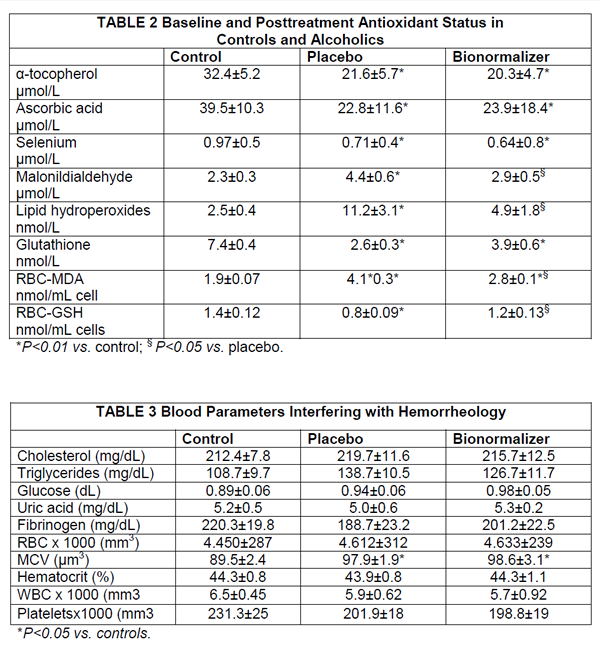
Serum concentration of bilirubin, albumin, prothrombin, cholinesterase, folate and cyanocobolamin were in the normal range in controls and in alcoholics. However, the latter group, no matter the further group allocation, comparably showed over 3- fold increase of aspartate aminotransferase (AST), alanine aminotransferase (ALT ) and γ-glutamyltransferase (γGT) and a raised MCV and serum acetaldehyde level, none of which was affected by antioxidant treatment. Further, baseline antioxidant assessment revealed a depleted status in alcoholics (Table 2). As compared to placebo, BN supplementation brought about a significant decrease of plasma level of malonyldialdehyde, lipid hydroperoxide and RBC-MDA (P<0.05, Table 2), all parameters being significantly elevated in alcoholics (P<0.05 vs. healthy controls) (Table 3). From routine blood chemistry, it appeared that the metabolic factors which would possibly affect hemorheological parameters did not show any difference between controls and placebo or BN-treated alcoholics (Table 3), As compared to controls, alcoholics showed a normal plasma viscosity but a significantly higher blood viscosity (P<0.05) (Figure 1) with an overall significantly lower whole blood filterability (P<0.05) (Figure 2). Dietary supplementation with BN brought about a significant improvement of the above abnormal parameters with a return to values comparable to control (P<0.01 vs. placebo). Although M and M1 values were comparable between controls and alcoholics (control M: 18.8±1.9, M1: 25.7±2.4; alcoholics M: 17.8±2.2, M1: 23.9±2.9), RBC aggregation index, as measured by disaggregation shear rate, was significantly decreased in the latter group when compared with healthy subjects (P<0.01) and this abnormality remained unaffected by the treatment (Figure 3). On the contrary, BN treatment reverted the significantly decreased RBC deformability observed in alcoholics (P<0.01 VS. controls) to normal values (P<0.05 vs. placebo). From the RBC membrane fluidity analysis no difference shown between controls and alcoholics, the motion parameter (expressing the rotational time in the hydrophobic region) was concerned (Figure 4). Antioxidant supplementation did not alter such parameters. However, alcoholics showed a significantly lower fluidity of the lipid bilayer closer to the
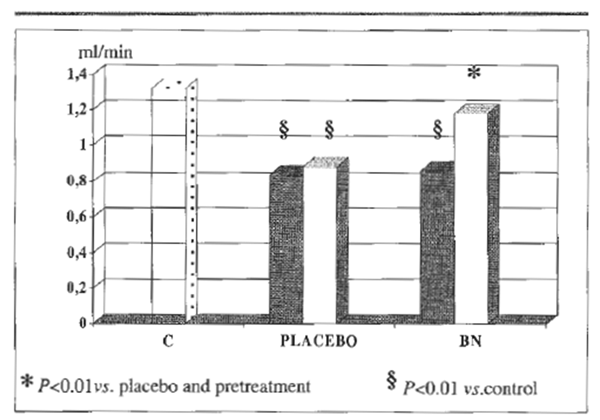 FIGURE 2. Whole blood filterability.
FIGURE 2. Whole blood filterability.
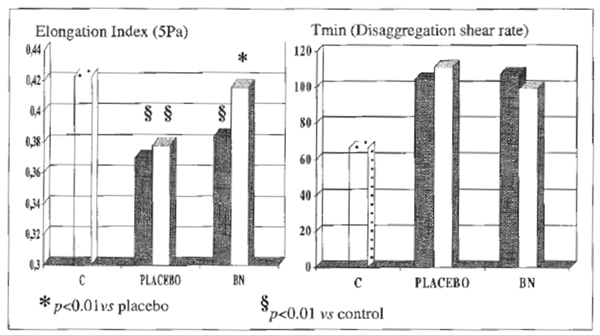 FIGURE 3 RBC-membrane deformity and RBC-aggregation index.
FIGURE 3 RBC-membrane deformity and RBC-aggregation index.
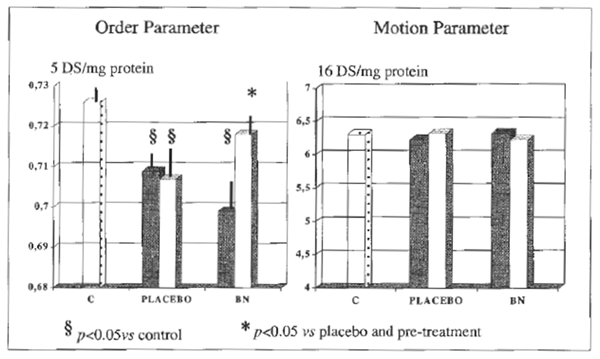 FIGURE 4 RBC-membrane fluidity.
FIGURE 4 RBC-membrane fluidity.
hydrophilic membrane side (expressed order parameter) (P<0.05 vs. controls) which was reverted to normal after BN addition to the diet. RBC-MDA was significantly correlated either with RBC deformability (r:0.62, P<0.05) and with membrane fluidity derangement (r:0 .58, P<0.05).
No further correlation appeared between rheological parameters and any of other abnormal biohumoral parameters.
DISCUSSION
Alcohol is metabolized in the liver and abnormal lipid metabolism, such as accumulation of triglycerides in the liver parenchyma has been already reported (20). Ln particular, chronic alcohol consumption in humans has been shown to affect the lipid composition of erythrocytes (21-23) whose cell membrane cholesterol to phospholipids ratio seems to be critical for membrane fluidity (22,24,25). On the other hand, microsomal hydroxyl radical products are generated in the liver during alcohol oxidation and increased serum level of malonyldialdehyde is frequently found in alcoholics (26). In turn, lipid peroxidation is known to markedly affect RBC surface characteristics and plasticity (27-29) and lipoperoxidation of RBC, besides that of hepatocytes, is likely to occur in alcoholics (30,31). In-deed, red blood cell population is one of the most susceptible tissues to be injured by exogenous free radicaIs (32) and it has recently been shown that proteins are oxidatively modified in plasma and erythrocytes in active alcoholics (33-35). Classically there are two main sources of oxygen free radicals which can damage RBC while altering also their rheologic properties: 1) internal generation, in the case of hemoglobinopathies; 2) extemal generation, in the case of ischemia-reperfusion injury or activated leukocytes. Therefore, alcohol seem to also act as an external oxidant and its oxidative effect might be further potentiated by the lack of a proper enzymanatic and none enzymatic antioxidant array which is often found in alcoholics (36), as was confirmed in the present study. Accordingly, Gerli et al. (37) have shown that RBC in patients with alcoholic liver cirrhosis have impaired antioxidant system. Although in our study the biochemical tests, known to influence hemorheological parameters, such as uric acid, cholesterol, triglycerides, glucose and fibrinogen, were comparable between teetotalers and alcoholics. The latter population showed a significantly higher blood, but not plasma, viscosity and impaired blood filterability. The relatively recent history of alcohol abuse in our population study with an overall preservation of the routine plasma lipid profile might probably account for the normal viscosity of plasma. When also considering the elevated RBC-MDA which were found in these patients, one could assume that a free radical-initiated damage to the RBC population is likely to be one of the major pathophysiological explanation of the rheologic abnormality observed. The whole blood filterability is an indirect index of erythrocyte deformability and microcirculatory flux (15) and, accordingly, RBC analysis showed that these cells exhibited a reduced deformability, which correlated with RBC-MDA (r:0.62, P< 0.05) and with membrane fluidity (r:0.58, P<0.05). Of interest, Bionormalizer supplementation, which brought about a partial, although significant improvement of a number of-oxidation parameters, reverted blood viscosity and filterability to normal. Further, it decreased the RBC lipoperoxidation which was paralleled by a decrease of RBC deformability with the polarity-correlation order parameter of membrane fluidity. The observer serum GSH depletion in alcoholics did not seem to benefit from antioxidant supplementation; however, RBC showed a restoration of GSH content whose importance in cell deformability has already been demonstrated in recent years (38). Loguercio et al. (39) have pointed out that alcoholic liver disease patients show a normal γ-glutamylcysteine synthese activity but a significantly depressed GSH activity. Therefore, it is unlikely that the antioxidant employed exerted its effect through the release of its substrates for GSH synthesis, such glutamic acid, glycine and methionine. The improvement of serum MDA and of lipid peroxides following antioxidant supplementation let us suggest that Bionormalizer, by reducing the systemic oxidative phenomena which are known to trigger either lipid and protein substrates modifications (33,34), might have influenced the cascade of events leading to a further damage of RBC population. Lndeed, such abnormalities are unrelated to a direct ethanol toxicity (33) and to acetaldehyde serum level which does not reflect the much higher concentration in RBC (40). Circulating xanthine oxidase released from injured cells in alcoholics is likely to play a contributory role in protein oxidation. Although in the present study we do not have any information on such a parameter, we have recently shown that the same antioxidant was able to significantly reduce ethanol-enhanced xanthine oxidase concentration in the gastric mucosa and restore GSH (8). Also of interest, γ-Tmin aggregation index, expressing the strength required for the minimal shear rate to fully disperse the RBC aggregates, was significantly elevated in alcoholics and did not improve under antioxidant therapy. Although we cannot provide data to clarify the pathophysiology of such derangement, the relationship between the fluidizing effect of alcohol together with the abnormality of the shaped of the physico-chemical pattern of RBC is likely to be involved. Further, we cannot expect which role platelets might have on this parameter in vivo, having been recently shown to undergo alterations of their chemical composition in alcoholics (41). RBC rheology abnormality presents multifaceted aspects to be investigated and the interplay with ongoing liver disease and membrane tolerance mechanisms still poses an unsolved controversial issue (2,42,43). Until recently, a great deal of investigations have analyzed the ethanol-induced modifications taking place in the RBC membrane lipid bilayer in chronic liver disease patients (44-49). Accordingly, a number of therapeutic approaches have been primarily directed toward the correction of inner RBC membrane lipid composition either in in vivo and in vitro studies (50-53). Nonetheless, although reactive oxygen species generated at different sites, i.e., external or internal to the RBC, might have different patterns of effect thus modifying the directionality of pathologic oxidant stress (32), the present study suggests that an effective antioxidant oral supplementation might prove to be a useful complementary tool in the therapeutic strategy. Such an approach is in agreement with the investigation from the group of Loguercio (54) in which a parenteral glutathione- donor treatment was able to correct the RBC thiol alteration, although no parallel rheological study had been performed. These overall preliminary results hold some interest when considering that, even after ethanol withdrawal, it could take a fairly long time to revert the RBC membrane fluidity impairment to normal (55-57). Moreover, it seems from clinical and experimental studies that RBC rheology derangement, in turn, might negatively affect hepatic microcirculation
at a sinusoidal level with a likely worsening of metabolic function (58-60).