| Title | BIOLOGICAL EFFECTS OF THE FERMENTATION PRODUCT OF CARICA PAPAYA (BIO-NORMALIZER) |
|---|---|
| Year | |
| Author | Hirotsugu Kobuchi,Nobuya Haramaki, Lucia Marcocci, Lester Packer |
| Publisher |
Biological Effects of the Fermentation Product of Carica papaya (Bio-Normalizer)
Hirotsugu Kobuchi,* Nobuya Haramaki+, Lucia Marcocci, + and Lester Packer+
*Environmental Energy Technologies Division
University of California Berkeley, California 94720
+ Membrane Bioenergetics Group Department of Molecular and
Cell Biology University of California Berkeley, California 94720
INTRODUCTION
Bio-Normalizer (BN) is a functional health food supplement sold in Japan, the United States, and in other countries. This product is made from Carica papaya Linn. And other plants (Pennisetum pupureum Schum Sechium edule Swartz) by yeast fermentation under strict quality control. Although ingredients of the product are still unknown in detail, carbohydrate (90%), protein, amino acids, and vitamins have been detected as main substances by chemical analysis (unpublished data). Bio-Normalizer has been proposed as a free radical modulating agent (Osato et al., 1995; Santiago et al., 1991). In biological studies, Bio-Normalizer has been found to scavenge hydroxyl radicals in vitro (Santiago et al, 1991), and in animal studies it has been reported to protect the rat brain against oxidative damage caused by aging (Santiago et al., 1993a), iron treatment (Santiago et al., 1992), or ischemia-reperfusion (Santiago et al., 1993b).From such reports, it has been proposed that beneficial effects of Bio-Normalizer might be due to its free radical scavenging properties. However, Bio-Normalizer has also been reported to upregulate phorbol ester-induced and zymosan-induced superoxide production in rat peritoneal macrophages (Osato et al., 1995), natural killer cell activity (Okuda et al., 1993), and the level of interferon (IFN-γ in human blood (Santiago et al., 1994). Such evidence suggests that BN also possesses the ability to modulate immune effector cells in addition to its direct free radical scavenging activity. Despite accumulating data on the beneficial effects of Bio-Normalizer, the biological mechanisms responsible for the therapeutic activity of Bio-Normalizer are not well understood. The authors have evaluated its activity under various conditions in order to gain further insight into the biological mechanisms of Bio-Normalizer action and new possibilities for therapeutic applications
ANTIOXIDANT PROPERTY OF BIO-NORMALIZER
Free radicals and other reactive oxygen species are formed constantly in the human body. They play a crucial role in a variety of human physiological functions. However, excess generation of reactive oxygen species can often give rise to oxidative stress those results from the imbalance in the human antioxidant/oxidant status. It has now been recognized that prolonging this imbalance is implicated in a number of human diseases (Halliwell, 1993). In fact, there is increasing evidence that reactive oxygen and nitrogen species are involved in the pathogenesis of a diverse range of chronic and degenerative disorders (aging, atherosclerosis, cancer, cataract), as well as in acute clinical conditions (ischemia-reperfusion injury) (Halliwell et al.,1989). Therefore, substances with antioxidant properties have been used as possible treatments of these disorders (Aruoma, 1994; Maxwell, 1995). To better define the antioxidant properties of Bio-Normalizer, BN was administered to rats for up to 6 weeks and the consequences of oral supplementation on in vitro models of oxidative stress-induced damage were then investigated
Myocardiac lschemia-Reperfusion Injury
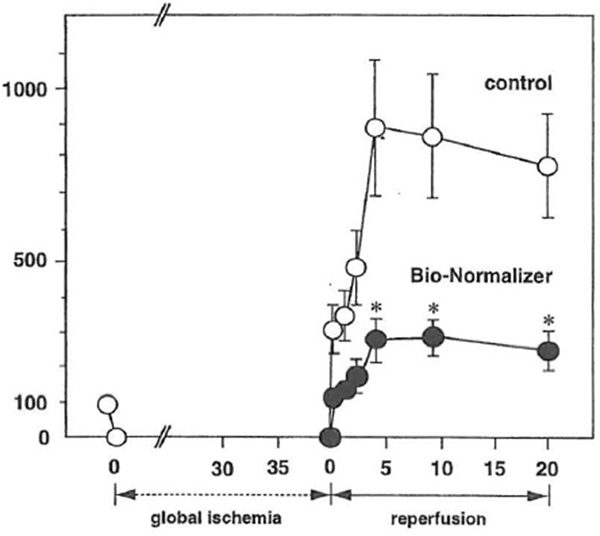
It is known that reactive oxygen species such as superoxide radical, hydroxyl radical and hydrogen peroxide are implicated in cardiac ischemia-reperfusion injury (Chan, 1996; Ferrari, 1995). Agents with free radical scavenging ability have been shown to enhance functional recovery of the heart exposed to ischemia-reperfusion (Janero, 1995; Singh et al., 1995). To delineate the antioxidant ability of BN against reactive oxygen radical-mediated injury, the influence of BN on cardiac ischemia-reperfusion injury was investigated (Haramaki et al., 1995). Male Sprague-Dawley rats (250-300 g) were fed a normal diet, supplemented with or without 0.1% BN (w/v) in drinking water. Six weeks later, the rats were subjected to 40 min of myocardial ischemia followed by 20 min of reperfusion using the Langendolff perfusion technique. Lactate dehydrogenase (LDH) leakage in the coronary effluent was monitored as an index of cardiac ischemia-reperfusion damage. During the reperfusion period, following 40 min of global ischemia, a high LDH leakage was observed in the effluent of the control rat heart, showing ischemia-reperfusion damage (Fig. 1). In contrast, BN-supplemented rat hearts showed a significantly lower level of LDH leakage (p < 0.01), suggesting that BN or its bioactive metabolites reached the rat heart through oral supplementation and prevented the hearts from ischemia-reperfusion injury.
Figure 1 Effect of Bio-Normalizer supplementation on leakage of LDH from the isolated Langendolff rat during reperfusion. LDH leakage into the coronary effluent from control (O) or Bio-Normalizer-supplemented hearts (●) was measured. LDH leakage of the preischemic period was expressed as 100%. Values are expressed as mean ± SEM obtained in six different experiments. *p < 0.01 compared with values from control hearts.
Peroxyl Radical-Induced Oxidative Stress
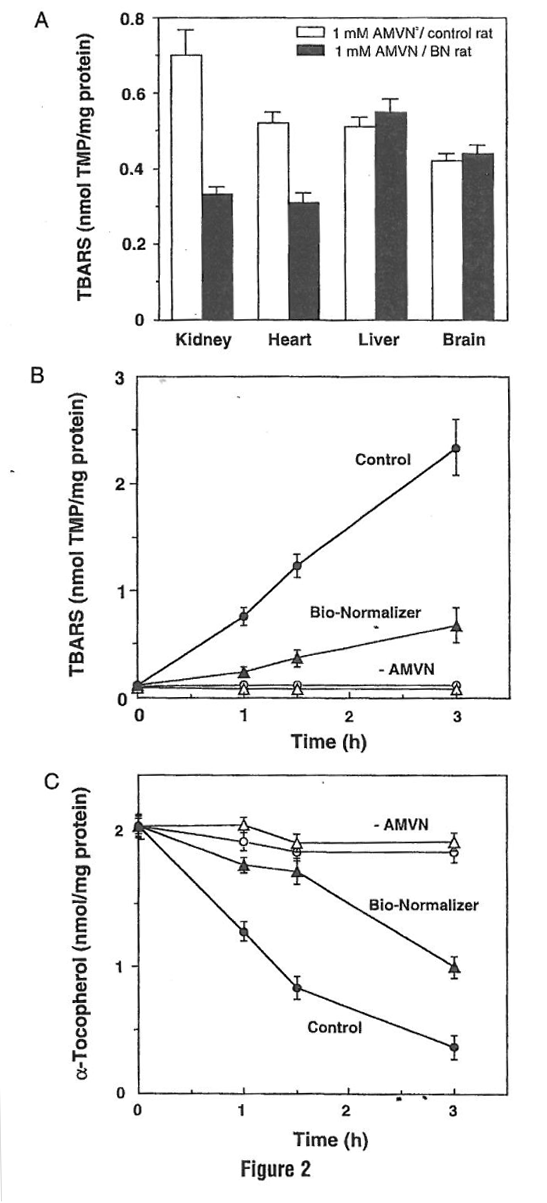
To further define the protective action of BN in rat heart and other tissues, the effect of BN supplementation on the susceptibility of various rat tissues to oxidative stress induced by peroxyl radials was analyzed (Marcocci et al., 1996). This radical, formed by the reaction of carbon-centered radicals with oxygen, has been known to be involved in the chain propagation step in lipid peroxidation (Halliwell and Gutteridge, 1989). The hydrophobic azo initiator [AMVN: 2, 2’-azobis (2, 4-dimethylvaleronitrile)] was used as a specific source of the peroxyl radicals generated at a constant rate on thermal decomposition of the azo initiator. The levels of thiobarbituric acid-reactive substance (TBARS) accumulation in homogenate samples were measured as an index of peroxyl radical-induced oxidative stress.
The level of TBARS in the absence of AMVN was very low in all homogenate samples (data not shown). In samples prepared from nonBN-supplemented animals, the exposure to AMVN resulted in higher levels of TBARS accumulation. In contrast, among the samples from BN-supplemented rats, significantly lower levels of TBARS were observed in kidney and heart (Fig. 2a). However, because the authors did not observe protection in either liver or brain homogenates, the uptake of BN to the tissue and its metabolism seems to be tissue specific. Moreover, oral supplementation of BN protected kidney homogenates from peroxyl radical-induced time-dependent accumulation of TBARS and depletion of α-tocopherol (Figs. 2b and 2c). These data further demonstrated BN to function as a free radical scavenger that protects the heart and cell membranes from free radical-induced oxidative damage. Thus one of the mechanisms in these protective effects of BN may be its antioxidant properties.
IMMUNOMODULATING ACTIVITY OF BIO-NORMALIZER
Nitric oxide (NO) is a versatile molecule that has many diverse biological functions. In addition to its function as a potent vasodilator and neurotransmitter, it is well established that NO plays a crucial role in the immune system as a cytostatic and cytolytic agent against tumorigenic cells (Nathan, 1992) or pathogenic organisms, including parasites (Green et al., 1990), viruses (Karupiah et al., 1993, 1995), and fungi (Granger et al., 1988). NO is synthesized by means of arginine oxidation by a family of nitric oxide synthases. Until now, three distinct isoforms of NO synthase (NOS, EC 1.14.13.39), neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS), have been isolated and characterized extensively (Nathan et al., 1994). Among these enzymes, eNOS contributes to the vasodilatation and adherence of platelets and leukocytes to endothelial cells; iNOS mainly participates in the host defense mechanism. Since the initial evidence of iNOS expression in murine macrophages was published, it has become apparent that iNOS is expressed by several cell types, including hepatocytes, endothelial cells, and smooth muscle cells. These cells have also been demonstrated to exert host defense activity by having iNOS activity in response to the stimulation of endotoxin and cytokines such as IFN-γ, interleukin-1β (IL-1β), and tumor necrosis factor- α (TNF-α) (Nathan, 1992).
Figure 2 Effect of Bio-Normalizer supplementation on AMVN-induced TBARS accumulation in various rat organ homogenates. (A) TBARS accumulation in various rat organ homogenates. (B) Time-dependent accumulation of TBARS in rat kidney homogenates. (C) Tune dependent depletion of α-tocopherol in rat kidney hornogenates. Rat tissues homogenates (10 mg protein/ml) prepared from control bars or from Bio-Normalizer-supplernented animals bars were incubated in PBS at 42°C for 1 h or at different periods in the absence or presence of 5 mM AMVN. Levels of TBARS and α-tocopherol were measured using 1, 1, 3, 3- tetramethoxypropane (TMP) as standard or the HPLC electrochemical method, respectively, and given as mean ± SEM of six different samples.
Direct Interaction with Nitric Oxide
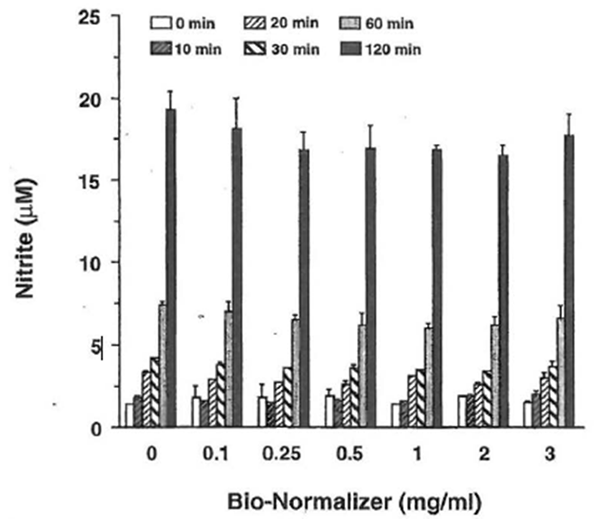
The authors demonstrated that BN scavenges oxygen radical species induced by either ischemia-reperfusion or a chemical system. In order to characterize the action of BN against NO radicals, the authors investigated whether BN interacted with NO directly. This evaluation was performed using the NO donor, sodium nitroprusside. This compound is known to decompose in aqueous solution at physiological pH to produce NO. Accumulated nitrite, which is the stable product of NO, was followed by reaction with the Griess reagent. The sodium nitroprusside solution was incubated with various amounts of BN (0-3 mglml) at 25°C for different time periods (0-120 min). BN had no effect on the level of nitrite accumulation in the reaction mixture {Fig. 3). This result suggests that BN itself
Modulation of Nitric Oxide Production in Macrophages by Bio–Normalizer
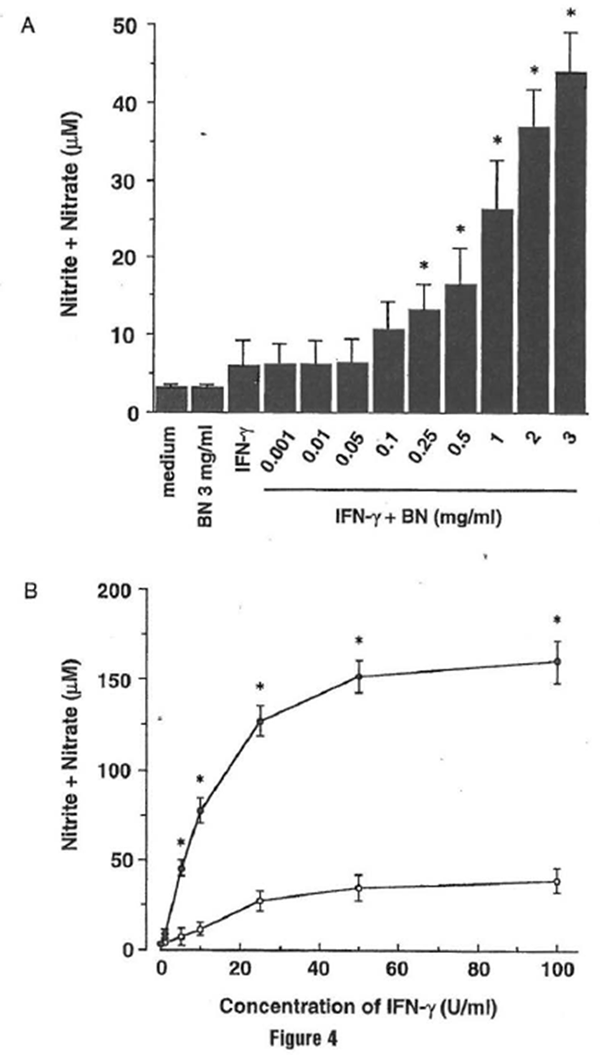
BN has been used for a variety of pathological conditions, and now BN has been reported to have beneficial effects against different diseases, such as cancer, hepatitis, and bacterial infection. BN has been reported to induce natural killer cell activity (Okuda eta/., 1993) and to enhance the capacity of respiratory burst in neutrophils (Osato et al., 1995). Such evidence encouraged us to evaluate BN as an immune modulator agent. The authors investigated the efficacy of BN to influence NO production in macrophages and characterized the mechanism by which BN affects cellular NO metabolism (Kobuchi and Packer, 1997). The mouse macrophage cell line RAW 264.7, which has been well characterized in NO studies and analyzed for the accumulation of nitrite/nitrate in culture medium using the Griess reagent as an index for NO synthesis, was used. Treatment with BN alone failed to induce an appreciable level of NO production. A major increase of NO production was observed when cells were treated with a combination of IFN-y plus BN; the enhancement of NO production by BN was dose dependent
(Fig. 4a). In addition, when macrophages were incubated with various concentrations of IFN-y, a similar effect on NO production was observed (Fig. 4b). IFN–y allows the production of appropriate levels of NO at concentrations above 5 U/ml. When 3 mg/ml BN was added into this test system, the IFN–y-induced NO production in macrophages was further upregulated by BN. These results suggest that BN itself dues not provide a signal that triggers induction of the NO pathway; however, BN possesses the ability to enhance the production of NO from activated macrophages.
In light of the unique properties of BN to act both as a direct free radical scavenger and as an upregulator of NO production, it was of interest to determine how it regulates cellular NO metabolism. In general, iNOS is modulated at different regulatory sites, including transcriptional, posttranscriptional, translational, and posttranslational control (Nathan and Xie, 1994). To characterize the mechanism by which BN upregulates IFN–y induced NO production in macrophages, the authors tested the effect of BN on the different steps that regulate NO production.
iNOS Enzyme Activity The ability of BN to affect the enzyme activity of iNOS directly was investigated (Fig. 5). NOS activity was determined by monitoring the conversion of [14C]arginine to citrulline using a cell-free preparation from activated macrophages as a source of iNOS. The direct modulatory action of BN on iNOS enzyme activity was not observed, even at high amounts of BN. These results suggest that the NO upregulation by BN is not because of its direct activation of iNOS enzyme activity.
Figure 3 Effect of Bio-Normalizer on NO produced by sodium nitroprusside. Sodium nitroprusside (5 mM) was incubated with various doses of SN as indicated in phosphatebuffered salin at 25°C. After incubation for different time periods, the aliquot of reactions was mixed with an equal volume of the Griess reagent and then the absorbance at 550 nm was measured. All values represent the mean ± SD of three independent experiments.
Figure 4 Bio-Normalizer synergizes with IFN- y for NO production in RAW 264.7 macrophages. (A) Dose-dependent effect of Bio-Normalizer on NO production. Macrophages were incubated with medium alone, Bio-Normalizer (3 mg/ml) alone, IFN-γ (5 U/ml) alone, or IFN-γ in the presence of the indicated concentrations of Bio-Normalizer for 24 hr. All values represent the mean ± SD of three independent experiments. *p < 0.05 compared with that of IFN-γ alone treatment. (B) Efficacy of IFN-γ on NO production by Bio-Normalizer. Macrophages were incubated with the indicated concentrations of IFN-γ in either the absence (○) or the presence (●) of 3 mg/ml of Bio-Normalizer for 24 hr. All values represent the mean ± SD of three independent experiments. *p < 0.05 compared with that of IFN-γ alone treatment.
iNOS mRNA Expression Macrophages did not express iNOS mRNA either constitutively or after treatment with BN alone. However, a low level of iNOS mRNA was observed following the stimulation of macrophages with IFN-γ alone. In contrast, treatment of macrophages with a combination of IFN-γ and BN resulted in a synergistic induction of iNOS mRNA, which was evident at a minimum concentration of 0.1 mg/ml BN (Fig. 6a). The augmentation was threefold greater than that induced by IFN-γ alone. These results were consistent with data on NO production, indicating that its synergistic interaction with IFN-γ to enhance NO production is due to the augmentation of iNOS mRNA expression. This iNOS gene upregulation effect of BN was also observed in a time-dependent manner (Fig. 6b). The IFN-γ-dependent induction in iNOS mRNA was not observed, even 2 h after stimulation. In contrast, the addition of 3 mg/ml BN caused a major increase of IFN-γ-induced iNOS mRNA expression that was already detectable after 2 h of treatment. After 6 h of incubation, BN caused about a threefold augmentation of iNOS mRNA expression over that induced by IFN-γ alone, similar to that observed in Fig. 6a. These data demonstrate that BN acted synergistically with IFN-γ in a dose- and time-dependent fashion for the induction of iNOS mRNA expression in macrophages.
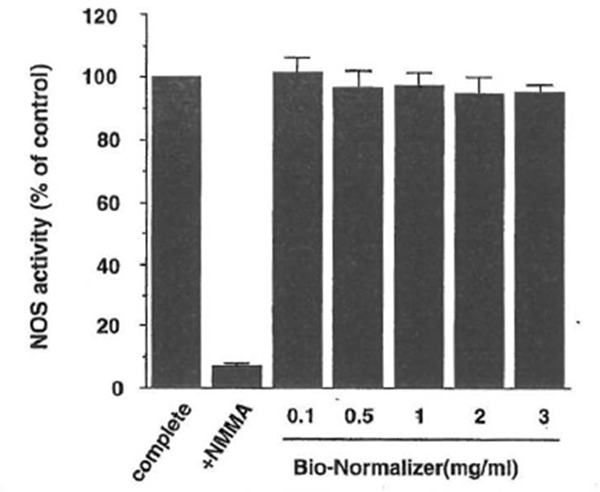
Figure 5 Bio-Normalizer does not affect iNOS enzyme activity. NOS activity was determined by the conversion of radiolabeled arginine to citrulline using a cytosolic preparation from macrophages. Complete indicates the value of iNOS activity in the reaction mixture that contains appropriate cofactors as a positive control. NG-monomethylarginine (NMMA) was present in the reaction mixture at 100 µ.M. Bio-Normalizer was added to the complete assay mixture as indicated. All values are expressed as a percentage of the control (100%: 178 ± 3 pmol/mg protein/min) and represent the mean ± SD of three independent experiments.
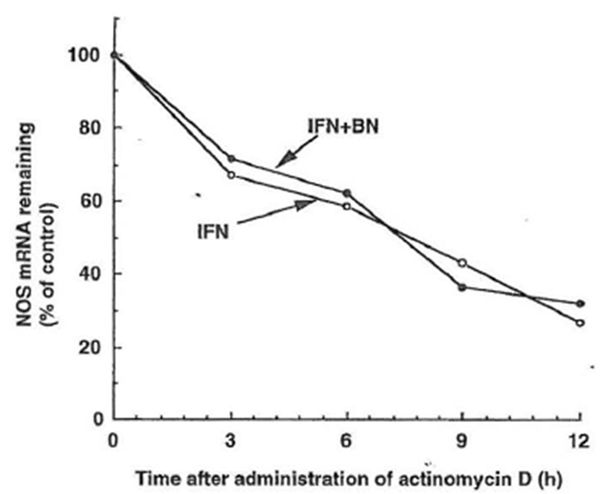
Stability of iNOS mRNA Upregulation of iNOS mRNA by BN is most likely caused by decreased mRNA destabilization and/or increased transcription rate. Weisz et al. (1994) reported that the half-life of iNOS mRNA in macrophages stimulated with a combination of LPS and IFN-γ was prolonged as compared to IFN-γ stimulation alone. In an attempt to determine the mechanism that may be involved in the augmentation of iNOS mRNA expression by BN plus IFN-γ, the stability of iNOS mRNA was measured using the de novo RNA synthesis inhibitor, actinomycin D. These experiments showed that there was no significant difference on iNOS mRNA stability between IFN-γ plus BN-treated cells and IFN-γ alone treated cells (Fig.7). These results indicate that the mechanism for augmentation of iNOS mRNA expression is not due to ‘an increase in the stability of iNOS mRNA) but rather to an increase in the transcription rate or other steps.
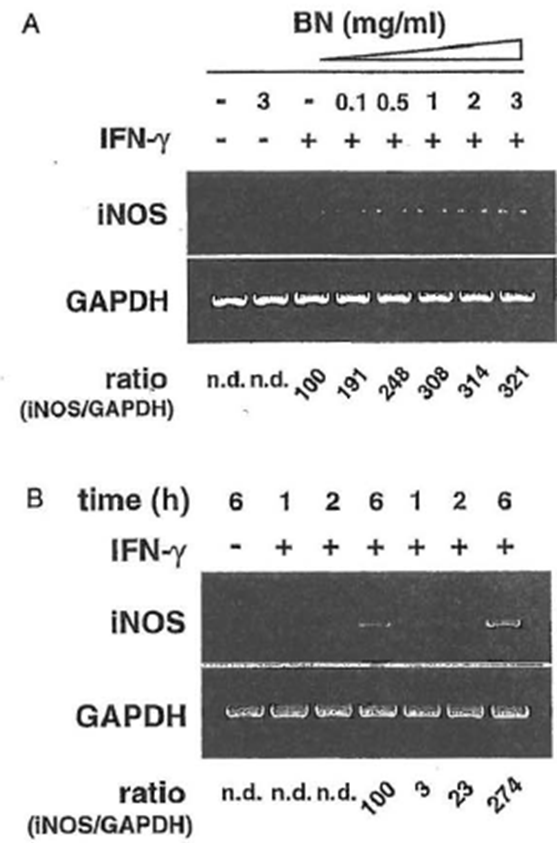
Figure 6 Synergistic induction of iNOS mRNA expression by IFN-γ plus Bio-Normalizer. (A) Dose-dependent induction. Macrophages were incubated with medium alone, BioNormalizer (3 mg/ml) alone, IFN-γ (5 U/ml) alone, or IFN– γ in the presence of the indicated concentrations of Bio-Normalizer for 6hr, and total cellular RNA was applied to RT-PCR using the iNOS-specific primer. All values are expressed as a percentage of the control (IFN-γ alone) for the iNOS/GAPDH ratio from results obtained by RT-PCR. (B) Time course induction Macrophages were incubated with IFN-γ (5 U/ml) alone or with IFN-γ plus BioNormalizer (3 mg/ml) for various periods as indicated, and total cellular RNA was applied to RT-PCR. All values are expressed as a percentage of the control (IFN-γ alone, 6 hr) for the iNOS/GAPDH ratio from results obtained by RT-PCR.
Figure 7 Effect of Bio-Normalizer on iNOS mRNA stability in IFN-γ -treated RAW 264.7 macrophages. Macrophages were incubated with IFN- γ (5 U/ml) alone (○) or with IFN-γ plus Bio-Normalizer (3 mg/ml) (●) for 6 hr. Actinomycin D (5 µg/ml) was then added to the culture, and total cellular RNA was extracted at the indicated time and applied to RT-PCR. Data are presented as the relative amount of iNOS mRNA remaining after the addition of actinomycin D and normalization to the respective amount of GAPDH mRNA.
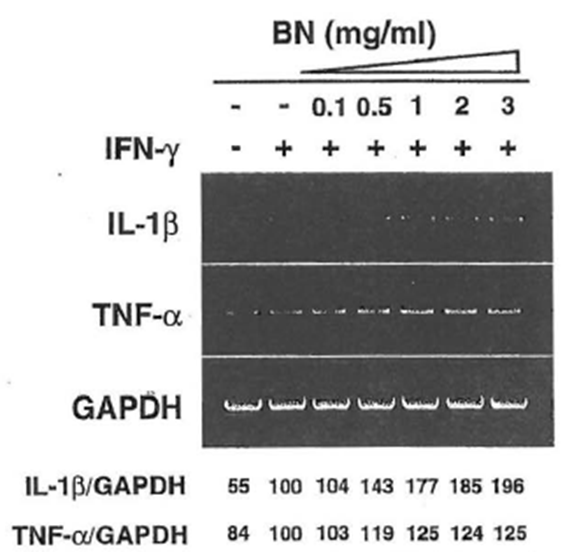
Upregulation of IL-1/3 and TNF-a mRNA Expression by Bio-Normalizer
It is well documented that the induction of iNOS gene expression is regulated tightly by cytokines (Oswald et al., 1996). For example, IFN-γ, TNFα, and IL-1β upregulate iNOS expression. Other cytokines, such as transforming growth factor-β, IL-4, and IL-10, have been shown to block the signal in the NO pathway. The ability of BN to enhance the expression of IL-β or TNF-α genes in macrophages was investigated because these cytokines are implicated in the induction of the iNOS gene as well as in tumoricidal activity. This macrophage cell line constitutively expresses IL-β and TNF-α mRNA, and these mRNAs were slightly enhanced by the treatment with IFN-γ (Fig. 8). In the case of IL-β, BN caused an approximately twofold augmentation over mRNA levels induced by IFN-γ alone. TNF-α mRNA levels were further enhanced by treatment with BN. These cytokines may be partially involved in the augmentation of iNOS mRNA expression in macrophages and in other cellular functions. Taking these results together, BN was not involved directly in the pathway for iNOS induction, but showed synergistic interaction with IFN-γ to induce NO synthesis in macrophages, suggesting that BN may have indirect mechanisms of microbicidal, tumoricidal activity because of its ability to modulate immune effector cells. In fact, macrophage-derived NO has been shown to induce hypoxia and apoptosis in tumor cells and to inhibit metastasis through a decrease in tumor cell-induced platelet aggregation. On the other hand, the release of high amounts of NO may damage healthy tissues and contribute to the pathogenesis of a wide range of diseases by the formation of peroxynitrite. However, the physiological function of NO appears to be determined primarily by the site and the quantity of its production. BN itself does not exert the upregulatory action on NO production unless iNOS inducers such as cytokines are present. Therefore, it would be interesting to investigate the antitumor effect in combined treatment of BN and IFN-γ in an animal model in order to understand further the mechanisms involved in the modulation of the immune system by BN. Further studies are needed to elucidate the bioavailability of BN to the human body, as well as the substances that are responsible for the free radical scavenging property and/or immunomodulating properties of BN.
Figure 8 Dose-dependent induction of IL-β, TNF-α mRNA by IFN- γ plus Bio-Normalizer. Macrophages were incubated with medium alone, IFN-γ (5 U/ml) alone, or lFN-γ in the presence of the indicated concentrations of Bio-Normalizer for 6 hr. Total cellular RNA was then extracted and applied to RT-PCR using IL-β or TNF-α-specific primers. All values are expressed as a percentage of the control (IFN- γ alone) from results obtained by RT-PCR.
REFERENCES
Aruoma, O. I. (1994). Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol. 32, 671-683.
Chan, P. H. (1996). Role of oxidants in ischemic brain damage. Stroke 27, 1124-1129. Ferrari, R. (1995). Metabolic disturbances during myocardial ischemia and reperfusion. Am.J. Cardiol. 76, 17B-24B.
Granger, D. L., Hibbs, J. B., Jr., Perfect, J. R., and Durack, D. T. (1988). Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J. Clin. Invest. 81, 1129-1136.
Green, S. J., Meltzer, M. S., Hibbs, J. B., Jr., and Nacy, C. A. (1990). Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism.J. Immunol. 144, 278-283.
Halliwell, B. (1993). The role of oxygen radicals in human disease, with particular reference to the vascular system. Haemostasis 23(Suppl. 1), 118-126.
Halliwell, B., and Gutteridge, J. M. (1989). “Free Radicals in Biology and Medicine.” Oxford University Press, Oxford.
Haramaki, N., Marcocci, L., D’Anna, R., Yan, L. J., Kobuchi, H., and Packer, L. (1995). BioCatalyzer α·r No. 11 (Bio-Nonnalizer) supplementation: Effect on oxidative stress to isolated rat hearts. Biochem. Mol. Bioi. Int. 36, 1263-1268.
Hogg, N., Kalyanaraman, B., Joseph, J., Struck, A., and Parthasarathy, S. (1993). Inhibition of low-density lipoprotein oxidation by nitric oxide: Potential role in atherogenesis. FEBS Leu. 334, 170-174.
Janero, D. R. (1995). Ischemic heart disease and antioxidants: Mechanistic aspects of oxidative injury and its prevention. Grit. Rev. Food Sci. Nutr. 35, 65- 81.
Karupiah, G., and Harris, N. (1995). Inhibition of viral replication by nitric oxide and its reversal by ferrous sulfate and tricarboxylic acid cycle metabolites. f. Exp. Med. 181, 2171-2179.
Karupiah, G., Xie, Q. W., Buller, R. M., Nathan, C., Duarte, C., and MacMicking, J. D. (1993). Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 261, 1445-1448.
Kobuchi, H., and Packer, L. (1997). Bio-Normalizer modulates interferon-y-induced nitric oxide
production in the mouse macrophage cell line RAW 264.7. Biochem. Mol. Bioi. Int. 43, 141-152.
Marcocci, L., D’Anna, R., Yan, L. J., Haramaki, N., and Packer, L. (1996). Efficacy of BioCatalyzer α·r No. 11 (Bio-Normalizer) supplementation against peroxyl radical-induced oxidative damage in rat organ homogenates. Biochem. Mol. Bioi. Int. 38, 535-541.
Maxwell, S. R. (1995). Prospects for the use of antioxidant therapies. Drugs 49, 345-361. Nathan, C. (1992). Nitric oxide as a secretory product of mammalian cells. FASEB ]. 6, 3051-3064.
Nathan, C., and Xie, Q. W. (1994). Regulation of biosynthesis of nitric oxide. f. Biol. Chem. 269, 13725-13728.
Okuda, H., Ominami, H., Zhou, A., Osato, A., and Santiago, L.A. (1993). Studies on biological activities of Rio-normalizer. Clin. Rep. 27, 4249-4258.
Osato, J. A., Korkina, L. G., Santiago, L. A., and Afanas’ev, I. B. (1995). Effects of bionormalizer (a food supplementation) on free radical production by human blood neutrophils, erythrocytes, and rat peritoneal macrophages. Nutrition 11, 568-572.
Oswald, I. P., and Stephanie, J. L. (1996). Nitrogen oxide in host defense against parasites. Methods 10, 8-14.
Santiago, L.A., Osato, J. A., Hiramatsu, M., Edamatsu, R., and Mori, A. (1991). Free radical scavenging action of Bio-catalyzer alpha.rho No. 11 (Bio-normalyzer) and its by-product. Free Radic. Biol. Med. 11, 379-383.
Santiago, L. A., Osato, j. A., Kabuto, H., and Mori, A. (1992). Decreased release of monoamine metabolites in iron-induced epileptogenic focus in the rat following administration of Bio-normalizer. Med. Sci. Res. 21, 139-1 41.
Santiago, L.A., Osato, J. A., Liu, J., and Mori, A. (1993a). Age-related increases in superoxide dismutase activity and thiobarbituric acid-reactive substances: Effect of bio-catalyzer in aged rat brain. Nettrochem. Res. 18, 711-717.
Santiago, L. A., Osato, J. A., Ogawa, N., and Mori, A. (1993b). Antioxidant protection of bio-normalizer in cerebral ischaernia-reperfusion injury in the gerbil. Neuroreport 4, 1031-1034.
Santiago, L.A., Uno, K., K.ishida, T., Miyagawa, F., Osata, J. A., and Mori, A. (1994). Effect of Bio-normalizer on serum components and immunological function in humans. Neurosciences 20, 149-152.
Singh, N., Dhalla, A. K., Seneviratne, C., and Singal, P. K. (1995). Oxidative stress and heart failure. Mol. Cell Biochem. 147, 77-81.
Weisz, A., Oguchi, S., Cicatiello, L., and Esumi, H. (1994). Dual mechanism for the control of inducible-type NO synthase gene expression in macrophages during activation by interferon- gamma and bacterial lipopolysaccharide: Transcriptional and post-transcriptional regulation. J. Biol. Chern. 269, 8324-8333.
Wink, D. A., Hanbauet; 1., Krishna, M. C., DeGraff, W., Gam”son, j., and Mitchell, j. B. (1993). Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl. Acad. Sci USA 90, 9813-9817.