| Title | EFFECT OF BIO-NORMALIZER ON WOUND HEALING IN EXPERIMENTAL RATS |
|---|---|
| Year | |
| Author | Allan L. Hilario, M.D., Maria Lourdes F. Angeles, M.D., and James Akira Osato, Ph.D. |
| Publisher |
EFFECT OF BIO-NORMALIZER ON WOUND HEALING IN EXPERIMENTAL RATS
By
Allan L. Hilario, M.D.,1 Maria Lourdes F. Angeles, M.D.,2 and James Akira Osato, Ph.D.2
1Department of Surgery, Ospital ng Maynila Medical Center, Manila
2Osato Research Foundation, Inc. Manila
Supervising Consultant:
Luis S. Montemayor, M.D., FPCS
Request reprints to: Dr. Lourdes Angeles, Osato Research Foundation
18/F Ayala Triangle Ayala Ave., Makati
ABSTRACT
The effect of Bio-Normalizer (BN), a potent free radical scavenger, on normal wound healing was investigated. Twenty adult male Sprague-Dawley rats underwent wounding on Day 0. The control group (n=10) received daily intraperitoneal (i.p.) injection of 1 ml 0.9% NaCl solution (NSS), while the experimental group (n=10) received daily i.p. injection of 1 ml BN solution (250 mg/ml) for 12 days. CBC and platelet count were determined on Days -4 and 12. Collagen synthesis was evaluated by collagen fiber diameter (CFD) measurement using electron microscopy of skin biopsy (Days 0, 6 and 12) and by collagen grading of skin biopsy (Days 0, 6, and 12). Statistical tests used were Student’s t test and Wilcoxon ranking technique.
The results showed a significant deposition of collagen on wounds of BN treated rats at Days 6 and 12 compared to NSS-treated rats (2.1 ± 0.57 vs. 1.8 ± 0.92, 3.3 ± 0.82 vs. 2.3 ± 0. 70 at Day 6 and 12 respectively, p < 0.05). CFD measurement revealed significant elevation in BN-treated rats at Day 12 (56 ± 9 vs. 52 ± 12 nm, p < 0.05). Significant reduction in CFD was noted in both groups from Day -4 to Day 12. Only in platelet count that a significant elevation was noted between groups.
In conclusion, BN enhanced collagen synthesis through its free radical scavenging action and proliferative effect on cells responsible for wound cytokine production.
Bio-Normalizer, Free Radical Scavenger, Wound Healing
INTRODUCTION
Wound healing is a complex biological process transforming injury into healed scar. Such process involves an orchestration of numerous cellular responses and a balance interaction of immuno-regulatory cells, tissue collagen synthesis and remodeling and angiogenesis. Evoking these various cellular responses are the main roles of various cellular components participating in wound repair. These cells such as macrophages, neutrophils, lymphocytes, platelets, keratinocytes, and many other blood and tissue cells, are responsible for the elaboration and synthesis of various cytokines which provide the necessary communication at the cellular level of wound healing.1
These cytokines which include Platelet-derived growth factor (PDGF),2-8,23 Transforming growth factor beta (TGFβ),2,6,8,1-12,23 Transforming growth factor alpha (TGFα),12,23 Epidermal growth factor (EGF),2,12,14-19,23 Tumor necrosis factor (TNF),20,21 and fibroblast growth factor (FGF),2,19,22,23 among others, have been studied individually in various combinations with other growth factors and in different concentrations to accelerate wound healing in both experimental animals and humans. These agents have potential clinical use and are the newer additions in the long list of agents in wound pharmacology.
In addition to this, free radical scavengers like dimethylsulfoxide and alpha-tocopherol are being employed to enhance cutaneous wound healing especially in free radical-mediated wound impairment.22 The involvement of these free radicals in various facets of pathologic wound healing has been studied extensively. However, the effect of free radicals on normal wound healing is yet unclear.
Bio-catalyzer α·ρ No. 11 (Bio-Normalizer) is a white, sweet, granular, natural health food commercially available in Japan and the Philippines and is made by yeast fermentation of Carica papaya Linn. (a widely known Philippine herb), Pennisetum pupureum Schum. (Napier grass), Sechium edule Swartz (vegetable) and glucose as main carbon source. It has been proven to possess potent scavenging action against oxygen free radicals especially hydroxyl radicals. 23-24
Since Bio-Normalizer (BN) was proven to have free radical regulating and modulating activity, this study investigated the effect of this biologically active compound on normal wound healing of experimental rats.
MATERIALS AND METHODS
Twenty healthy adult male Sprague-Dawley rats weighing 300 – 350 grams were obtained from the Bureau of Animal Industry, Alabang, Philippines. The animals were housed in an animal laboratory in a Manila university. The animals were kept in aluminum cages (5 rats/cage) and a 12-hour light and dark cycle was observed. Food and water were supplied ad libitum. The experimental animals were taken cared of in accordance with the guidelines set by the institutional committee regarding studies employing the use of experimental animals.
Bio-Normalizer was supplied by Fil. Sun-O International, Inc. Manila, Philippines. Distilled water was used as solvent for BN.
Two groups of rats were formed. One group served as the control group which received daily intraperitoneal injection of 1 ml 0.9% NaCl solution for 12 days while the other group served as the experimental group which received daily intraperitoneal injection of 1 ml 250 mg/ml Bio-Normalizer solution for 12 days.
On the day of surgical wounding (Day 0), all rats were anesthetized with 4 mg/kg Thiopental Sodium (Penthotal Sodium, Abbott-Metro Laboratories, Inc. Manila, Philippines) intraperitoneally. The abdomen was shaved and a midline vertical incision was done and carried through the panniculus carnosus 4 cm in length. The incision was closed by simple interrupted suture using Silk 4-0. No dressing was applied and the surgical wounding was performed under strict asepsis and anti-sepsis. Sutures were removed on the 3rd day post-wounding without anesthesia.
Preparation for light microscopy and semi-quantitation of collagen deposition
On Day 0, skin biopsy measuring 4 mm x 4 mm perpendicular to the wound was taken at the cephalad end which served as the baseline sample. Subsequent biopsies were taken at the middle and caudal end of the wound for Day 6 and Day 12, respectively. All biopsies were done under Thiopental anesthesia. The skin samples were fixed in 10% phosphate-buffered formaldehyde. The samples were divided into two. One half was stained with hematoxylin and eosin. The other half was stained with specific stain for collagen fibers using the methods of Von Gieson and Weigert.25 Two independent dermatopathologists examined the slides in a coded fashion. An arbitrary scale of Grade 1- 4 was used to assess semi-quantitatively the degree of collagen synthesis and fibroblast cellularity (Table 1). The average of the two observers was used as the grade assignment.
Preparation for electron microscopy and collagen fiber diameter measurements
Skin biopsy was also collected at Day 0 and Day 12 from similar incision on all rats measuring 4 mm x 4 mm and perpendicular to the long axis of the incision scar. The collected specimen was minced and fixed in 2.5% glutaraldehyde in 0.1M phosphate buffer (pH 7 .4) for 24 hours, post-fixed in 1% OsO4 in distilled water for 1 hour, then dehydrated through epoxy resin. Test areas from where collagen fiber diameter measurements were made were identified through light microscopic examination of one micron plastic sections in order to specifically exclude adjacent normal dermis. Then, 100 run (silver) sections were cut on a LKB ultramicrotome, stained with lead citrate and uranyl citrate and photographed by using an EM “HU-RA” (magnification x 12,000) and obtained negatives were additionally enlarged by 16 times. EM magnification was standardized by using replica of diffraction-grate. Collagen fiber diameter (CFD) measurements were taken from 50 collagen fibers from each specimen and carried out close to an active fibroblast. All collagen fibers measured from each specimen taken from each group were included for morphometric and statistical analysis. Sample preparation and embedding were performed at the Natural Science Research Institute, University of the Philippines, Diliman, Quezon City, Philippines. After completion of specimen collection and preparation, the embedded samples were sent in coded labels for electron microscopy.
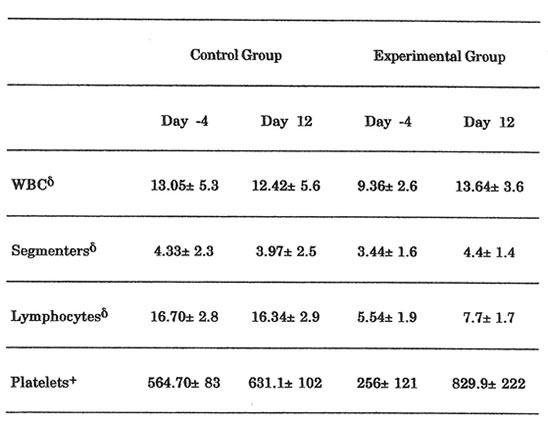
Blood samples were collected at Day -4 and Day 12 before collecting the last skin biopsy using EDTA as anticoagulant. The samples were subjected to complete blood count and platelet count determinations using an automated cell counter (Serono Systems 9000+, Baker Instruments, Allentown, Pennsylvania). Absolute counts of lymphocytes and segmenters were determined using the formula: Absolute cell count (1 x 109/L) = WBC count x% Differential.
The results of all hematologic parameters and CFD were reported as mean ± SD. The intra-group and inter-group analyses for statistical significance were performed using the Student’s t-test. Since the results of collagen staining were in ordinal number, the non-parametric test for statistical significance was performed using the Wilcoxon Ranking Technique. The level of significance used was set at p< 0.05.
RESULTS
Tissue sections stained with hematoxylin and eosin showed perceptible differences at the light microscopic level between BN-treated and NBS-treated rats. Inflammatory changes such as WBC infiltrates, edema and congestion, in BN-treated rats were minimal at Day 6. However, inflammatory change parameters were noticeably lesser in BN-treated rats at Day 12. Mild to moderate inflammation was evident in NSS-treated rats at Day 0 continuing thru Day 12 at minimal levels.
Collagen deposition and fibroblast cellularity, as stained with Weigert’s and Von Gieson’s, in wounds of BN-treated rats were significantly improved over those of the NSS-treated rats. The mean grade of BN-treated rats using the collagen staining scale was 2.1 ± 0.57 at Day 6 as compared to 1.8 ± 0.92 in NSS-treated rats (p< 0.05). Similar findings were noted at Day 12 where the mean grade of BN-treated rats was 3.3 ± 0.82 while the NSS-treated rats was 2.3 ± 0. 70 (p<0.05) (Table 2).
Electron microscopic findings showed no significant difference in fibroblast morphology between the two groups. Fibroblasts taken from both groups showed similar characteristics and activity. Initial measurement of CFD in both groups showed no significant difference at Day 0. This connotes that samples taken from each group showed homogeneity. However, a significant decrease in CFD measurement was noted in both groups between Day 0 and Day 12 samples (p<0.05). Significant difference was noted in the CFD measurement between the control and experimental groups at Day 12. Mean CFD in experimental group was significantly greater than the control group (56± 12 vs. 52.1 ± 12 nm, p < 0.05). Some samples were found to be poorly prepared and staining with lead citrate and uranyl citrate for electron microscopy were not carried out. Such samples were discarded and were not included in the CFD measurements (Table 3).
Significant elevation in the platelet count was noted in BN-treated rats from Day -4 to Day 12 (p < 0.05). Also, the BN-treated rats showed significant elevation in platelet count when compared to the NSS-treated rats (p < 0.05). Intra-group and inter-group analyses of WBC and absolute counts of segmenters and lymphocytes showed similar trends but the differences noted were not significant (p > 0.05, Table 4).
DISCUSSION
Normal wound healing proceeds through an orderly cascade of events which are geared towards creating a normal structure-function relationship.26 Such process of wound healing occurs in varying time frames, depending upon the length of these cellular events, how orderly such interplay occurs and the different factors affecting wound repair and healing in general.
These factors affecting wound healing and repair have been studied extensively in the past. But little is understood about the effect of oxidative stress in normal wound healing. The injurious effects of oxygen radicals to cutaneous structures have been well-documented and occur in various skin conditions such as damage by ultraviolet radiation and the phototoxicity of porphyria, certain tetracyclines and benoxaprofen.27
However, oxygen free radical production in mammalian systems is not at all deleterious. Such active oxygen species can also provide these cellular systems with very important physiological properties. They can provide defense mechanism against exogenous bacterial contamination,28,29 serve as adaptogenes by inducing endogenous free radical defense systems30 and stimulate growth, maturation and differentiation in a variety of mammalian cell.31 Thus, the ability of oxygen free radicals to cause injury to mammalian cellular systems does not depend on the level of active oxygen species present but on the absence or presence of balance in the organism’s free radical homeostasis.
Normal wound healing process of surgical incision is not at all innocuous. Initial response to a surgical injury is a clot with platelet aggregation and activation followed by release of alpha-granules, which contain PDGF and TGF-beta and other chemotactic factors. These cytokines and wound growth factors are chemo attractant to the PMN leukocytes which migrate into the local wound area and start the phagocytosis of local clots.26 In the process of phagocytosis of local clots and cellular debris, these PMN leukocytes release superoxide radicals.32 As more and more PMN leukocytes are recruited into the wound site, more superoxide radicals are formed and the homeostatic balance of free radicals in the body is deranged which can exert injurious potential on other cellular mechanisms involved in wound healing and repair.
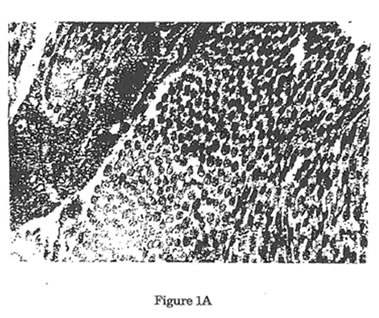
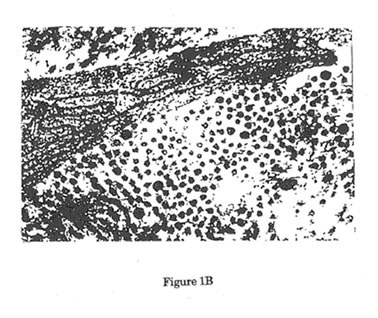
In this study, as seen also in the study of Devereux et al,33 surgical wounding produced significantly collagen fibers with smaller diameter than that seen in unwounded skin (Figure 1). Such failure to produce collagen fibers with similar diameters as in unwounded normal skin is responsible for the inability of normal wound healing mechanism to achieve the functional characteristics of an intact skin. The reason behind this is yet unclear. But we can only hypothesized that in this model of normal wound healing where other factors for wound impairment are absent, the inherent oxidative stress brought about by the inflammatory challenge secondary to the surgical incision caused the significant attenuation of the normal healing potential.
The cytotoxic oxygen species produced by PMN leukocytes such as superoxide radical and hydrogen peroxide can affect wound healing directly and indirectly. Collagen, together with other extracellular matrix structural macromolecules like proteoglycans and elastin are easily degraded by oxygen radical attack.34,35 Such damage can be potentiated by the activation of latent collagenases by oxidants causing further degradation of collagen.
Indirectly, the significant levels of oxygen free radicals achieved during the inflammatory phase may be cytotoxic to other cellular components in wound healing like the platelets, WBC and macrophages which are rich sources of local wound cytokines and growth factors. They provide the important proliferative signals to fibroblasts and epidermal cells to perform their synthetic and regenerative functions to promote wound healing.26
In order to elucidate the effects of free radicals in wound healing, Korkina et al [unpublished data], measured the level of free radical production in experimental rats at 3-day interval using Luminol- and Lucigenin-dependent chemiluminescence technique. Normal rats surgically wounded gave higher value on spontaneous and latex-activated chemiluminescence reactive up to the 10th day post-wounding. Maximal production of oxygen free radicals corresponds to the inflammatory response. During inflammation, PMN leukocytes produce significant release of oxygen free radicals which serve as chemoattractants thereby affecting more PMN leukocytes. This response was observed on the 3rd day post-wounding and continuously increased until the 10th day. As the inflammation subsides, the level of free radical generation starts to decline until it reaches its previous level prior to wounding.
However, biologically active substances can influence oxygen radical production. These agents can inhibit neoplastic events at the cellular and molecular levels,37 decrease the damages induced by ischemia-reperfusion,38,39 diminish the inflammatory changes,40 and prevent various environment associated pathology.41 The therapeutic and preventive action of such agents may be due to the regulation and maintenance of a normal free radical status in the organism.
BN is a complex mixture of biologically active components (yeast cell, glucose, antioxidant enzymes and amino acids) proven to have both inhibitory and stimulatory effects on free radicals.42 Thus, the balance of oxygen free radicals achieved in the wound site could enhance significantly the process of wound healing.
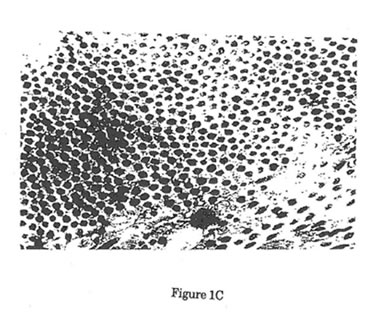
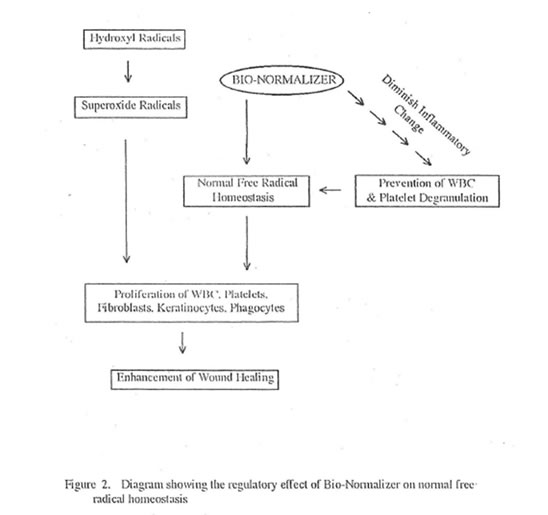
In this study, BN enhanced wound healing by enhancing collagen synthesis and deposition as measured semi-quantitatively using specific collagen staining methods and quantitatively using collagen fiber diameter measurement by electron microscopy. The ability of BN to enhance wound healing is due to its regulatory effect on normal free radical homeostasis (Figure 2). This regulatory effect is secondary to its ability to quench free radicals directly, convert hydroxyl radicals into innocuous superoxides, diminish the inflammatory change and delimit sources of free radical production. Lastly, the homeostatic balance of free radicals especially that of superoxides enhances the proliferation of various mammalian cells participating in wound healing such as phagocytes, leukocytes, platelets, fibroblasts and keratinocytes.
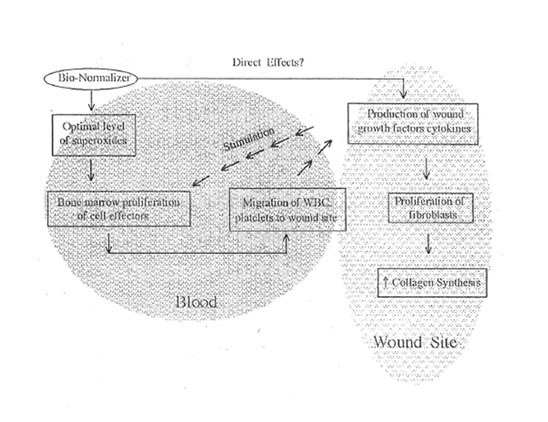
Bio-Normalizer as shown in this study caused these cells to proliferate markedly although the results were only significant for platelets. The proliferation of these cells is brought about by the normalizing activity of BN on the level of superoxides in the blood and other tissues. It was shown that the optimal level of superoxides can cause bone marrow cell proliferation.42 This is in turn responsible for the increased production of WBC and platelets which are rich in cytokines and growth factors (Figure 3). However, the direct effect of BN on the wound cytokines and growth factors is still under laboratory investigation.
CONCLUSION
In this study, the oxidative stress involved in normal wound healing as inflicted by surgical incision in experimental rats was characterized and the effect of Bio-Normalizer, a proven agent with regulating activity on free radical production was investigated. Bio-Normalizer enhanced collagen synthesis and deposition in normal process of wound healing in experimental rats through its modulating and regulating activity on free radical production and proliferative effect on cellular components participating in normal wound healing.
REFERENCES
1. Cohen IK, Diegelmann RB, Crossland MC. Wound care and wound healing, In: Schwartz Sl, Shires GT, Spencer FC, Busser WC, eds. Principles of Surgery. 6th edition, New York, McGraw-Hill. 1994; 279-300.
2. Sprugel KH, McPherson JM, Clowes AW, Ross R. Effects of Growth Factors in vivo, I: Cell ingrowth into porous subcutaneous chambers. Am J Pathol 1987; 129(3):601-613.
3. Narayanan AS, Page RC: Biosynthesis and regulation of type V collagen in diploid human fibroblasts. J Biol Chem 1983; 258(19): 11649-11699.
4. Chua CC, Geiman DE, Keller GH, Ladda RL. Induction of collagenase secretion in human fibroblasts cultures by growth promoting factors. J Biol Chem 1985; 260(9): 5213-5216.
5. Mustoe TA, Landes A, Cromack DT, et al. Differential acceleration of healing of surgical incisions in the rabbit gastrointestinal tract by platelet-derived growth factor and transforming growth factor, type beta. Surgery 1990; 108(2): 324-329.
6. Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Plateletderived growth factor in chemotactic for fibroblasts. J Cell Bio 1982; 92(2): 584- 588.
7. Deuel TF, Senior RM, Huang JS, Gri1fan GL: Chemotaxis ofmonocytes and neutrophils to platelet-derived growth factor. J Clin Invest 1982; 69(4): 1046-1049.
8. Lawrence WT, Sporn MB, Gorschboth C, Norton JA, Grotendorst GR. The reversal of an Adriamycin Induced Healing impairment with chemoattractants and growth factors. Ann Surg 1986; 203(2): 142-14 7. 12
9. Postletthwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. JExpMed 1987; 165(1): 251-256.
10. Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc NatlAcad Sci USA 1986; 83(12):4167-4171.
11. Ignot RA, Massague J. Transforming growth factor-f\ stimulates the expression of fibronectin and collagen and their incorporation in the extracellular matrix. J Biol Chem 1986; 261(9):4337 -4345.
12. Carpenter G, Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J CeU Physio 1975; 88: 227-238.
13. Buckley A, Davidson JM, Kamerath CD, Wott TB, Woodwad SC. Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci USA 1985; 82(21):7340-7344.
14. Niall M, Ryan GB, O’Brian BM. The effect of epidermal growth factor on wound healing in mice. J Su111 Res 1982; 33(2):164-169.
15. Brown GL, Curtsinger L. Brightwell JR. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med 1986; 163(5): 1319-1324.
16. Laato M, Niinikoski J, Lundberg C, et al. Effects of epidermal growth factor (EGF) on experimental granulation tissue. J Surg Res 1986; 41(3): 252-255.
17. Davidson J, Buckley A, Woodward S, et al. Mechanisms of accelerated wound repair using epidermal growth factor and basic fibroblast growth factor. Prog Clin Bi.ol Res 1988; 266:63-7 5.
18. Le J, Vilcek J. Tumor necrosis factor and interleukin 1: Cytokines with multiple overlapping biological activity. Lab Invest 1987; 56(3):234·248.
19. Osler W, Lindenmann A, Hans, et al. Tumor necrosis factor (TNF) alpha but not TNF-beta induces secretion of colony stimulating factor for macrophages (CSF-1) by human monocytes. Blood 1987; 70: 1700-1706.
20. Montesano R, V assalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA 1986; 83(19):7297-7301.
21. Ford HR, Hoffman RA, Wing EJ, Magee DM, Mcintyre L, Simmon RL. Characterization of wound cytokines in the sponge matrix model. Arch Surg 1989; 124(12): 1422-28.
22. Svingen BA, Powis G, Appel PL, Scott M. Protection against adriamycininduced skin necrosis in the rat by dimethyl sulfoxide and alpha-tocopherol. Can Res 1981; 41(9 Pt 1): 3395-3399.
23. Santiago LA, Osato JA, Hiramatsu M, Edamatsu R and Mori A. Free radical scavenging action of Bio-catalyzer a.p No. 11 (Bio-Normalizer) and its by product. Free Rad Biol Med 1991; 11: 379-383.
24. Santiago LA, Osato JA and MoriA. Stability of hydroxyl radical scavenging components of the food supplement “Bio-Normalizer”. Med Sci Res 1992; 20: 27-28.
25. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd edition, McGraw-Hill, Inc. 1968:35-36.
26. Lee RC, Doong H. Control of matrix production during tissue repair. In: Anderson DK, Lee RC, Mustoe TA, Siebert JW, eds. Advances in wound healing and repair. Master Series in Surgery Vol. 4, New York, World Medical Press, 1993; 1-25.
27. Halliwell B, Borish ET, Pryor WA, et al. Oxygen radicals and human diseases. [Clinical Conference] [Review]AnnlntMed 1987; 107(4): 526-545. 14
28. Babior BM, Kipnes BS, Curnette JT. Biological defense mechanis DL The production by leukocytes of superoxides, a potential bactericidal agent. J Clin Invest 1973; 52(3): 741-744.
29. Forman HJ, Thomas NJ. Oxidant production and bactericidal activity of phagocytes. [Review J Ann Rev Physiol 1986; 48: 669-680.
30. Davies JM, Lowry CV, Davies KJ. Transient adaptation to oxidative stress. in yeast. Arch Biochem Biophys 1995; 317(1):1-6.
31. Hamilton JA, Veis N, Bordun AM, Vairo G, Gonda TJ, Phillips WA. Activation and proliferation signals in murine macrophages: Relationship among c-fos and c-myc expression, phosphoinositide hydrolysis, superoxide formation and DNA synthesis. J Cell Physiol 1989; 141(3): 618-626.
32. Fridovich I. Superoxide radical: an endogenous toxicant. Anri Rev Pharmacol Toxicol1983; 23: 239-257.
33. Devereux DF, Triche TJ, Webber BL, Thibault LE, Brennan MF. A study of adriamycin-reduced wound breaking strength in rats. An evaluation by light and electron microscopy, induction of collagen maturation, and hydroxyproline content. Can 1980; 45(11): 2811-2815.
34. Babior BM. Oxygen-dependent microbial killing by phagocytes, part I. N Engl J Med 1978; 298(12):659-668.
35. HalliwellB, Gutteridge JM. The importance of free radicals and catalytic metal ions in human diseases [Review]. Mole Aspect Med 1985; 8(2): 89-193.
36. Perchellet JP, Perchellet EM. Antioxidants and multistage carcinogenesis in mouse skin. [Review]. Free Rad Biol Med 1989; 7( 4): 377 ·408.
37. Simpson PJ, Lucchesi BR. Free radical and myocardial ischemia and reperfusion injury [Review]. J Lab Clin Med 1987; 11 0(1): 13-30.
38. Lesnefsky EJ. Reduction of infarct size by cell permeable oxygen metabolite scavengers [Review]. Free Rad Biol Med 1992; 12(5):429-446.
39. Vapaatalo H. Free radicals and anti-inflammatory drugs. Med Biol 1986;64(1): 1-7.
40. Ames BN. Dietary carcinogens and anticarcinogen: Oxygen free radicals and degenerative diseases [Review]. Science 1983; 221(4617): 1256-1264.
41. Osato JA, Afanas’ev m, Cheremisina ZP, et al. Effects of Bio-Normalizer on free radical production by human blood erythrocytes, neutrophils and rat peritoneal macrophages. Nutrition: Intl J of Appl and Bsic Nutritional Sci. 1995;1(5):1-10.
42. Murrell GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J 1990; 265(3):659-665. 16
APPENDIX
Table 1. Scale to assess the collagen synthesis and fibroblast cellularity
| GRADE MICROSCOPIC DESCRIPTION |
| 1 Minimal collagen deposition and fibroblast cellularity |
| 2 Moderate collagen deposition and fibroblast cellularity |
| 3 Marked collagen deposition and fibroblast cellularity |
| 4 Maximal collagen deposition and fibroblast Cellularity |
Table 2. Results of Collagen Staining δ
| Day 0 | Day 6 | Day 12 | |
| Control Group | 1.6± 0.64 | 1.8± 0.92 | 2.3± 0.70 |
| Experimental Group | 1.9± 0.36 | 2.1± 0.57 | 3.3± 0.82 |
δReported using the scale in Table 1
Intra- and intergroup analyses showed statistical significance between groups on all days of observation except Day 0
Table 3. Collagen Fiber Diameter Measurement
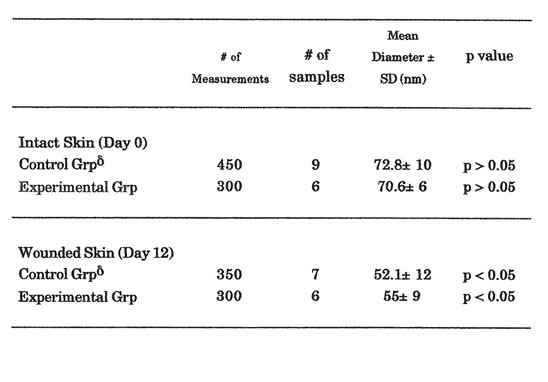
δThe decrease noted in the collagen fiber diameter measurements between intact and wounded skin was significant (p < 0.05)
Table 4. Results of Hematological Parameters
Only in platelet count that a statistically significant elevation between the 2 groups was noted (p<0.05)
Figure1. Electron micrographs of collagen fibers cut in cross-section. (12,000x,16x) Uranyl acetate/Lead Citrate Stain. A Representative area of collagen fibers taken from unwounded control rats. Predominance of large mature collagen fibers was noted. B. Representative area of collagen fibers from wounded control rats 12 days post-wounding. Predominance of small immature collagen fibers was noted with significant reduction of mean rats. Such reduction in the size of collagen fibers after surgical incision is the reason behind the failure of the normal skin to achieve its pre-wounding tensile strength. C. Representative area of collagen fibers from wounded experimental rats 12 days after treatment with Bio-Normalizer. Such treatment showed significant elevation in the size of collagen fibers when compared with wounded control rats.