| Title | BIO-NORMALIZER MODULATES INTERFERON-γ-INDUCED NITRIC OXIDE PRODUCTION IN THE MOUSE MACROPHAGE CELL LINE RAW 264.7 |
|---|---|
| Year | 1997 |
| Author | Hirotsugu Kobuchi and Lester Packer |
| Publisher | Biochemistry and Molecular Biology International |
BIO-NORMALIZER MODULATES INTERFERON-g-INDUCED NITRIC OXIDE PRODUCTION IN THE MOUSE MACROPHAGE CELL LINE RAW 264.7
Hirotsugu Kobuchi1 and Lester Packer*
1 On leave from Institute for Medical Science, Center for Adult Disease, Kurashiki 710, Japan
* Corresponding author, Energy and Environment Division, Lawrence Berkeley National
Laboratory, University of California, Berkeley, California 94720-3200
Energy and Environmental Division, Lawrence Berkely National Laboratory,
University of California, Berkely, Califonia 94720-3200
SUMMARY Bio-Normalizer, a natural health food supplement prepared from Carica papaya and some other medicinal plants was investigated to determine its effects on cellular nitric oxide (nitrogen monoxide, NO) production and inducible nitric oxide synthase (iNOS) expression. Bio-Normalizer upregulated interferon (IFN)-g-induced NO production by macrophages in a dose-dependent manner. Such an effect of Bio-Normalizer on NO production was not due to changes in the activity of iNOS. Reverse transcription-polymerase chain reaction analysis revealed that the levels of iNOS mRNA were augmented by treatment of the cells with Bio-Normalizer and IFN-g. The ability of Bio-Normalizer to augment IFN-g-induced iNOS mRNA expression was independent of any changes on the mRNA stability. Treatment of cells with Bio-Normalizer alone did not affect NO production by macrophages. Tumor necrosis factor-a and interleukin-1b are involved in the induction of iNOS gene as well as the immune system. Bio-Normalizer augmented the mRNA expression of these cytokines in the presence of IFN-g. This suggests that Bio-Normalizer is not directly involved in the expression of iNOS, but shows synergistic interaction with IFN-g to induce NO synthesis.
Key words: nitric oxide , inducible nitric oxide synthase, Bio-Normalizer, RAW 264.7
INTRODUCTION
Macrophages play a significant role in the host defense mechanism by the secretion of various cytotoxic agents, including lysosomal enzymes, reactive oxygen species and reactive nitrogen intermediates (1) (2). Among the reactive nitrogen intermediates, nitric oxide (nitrogen monoxide, NO) is one of smallest active biological metabolites, with a short half-life in the order of seconds (3). Recent studies have shown that the NO is associated with the expression of microbicidal and tumoricidal activities exerted by macrophages (2). Furthermore, it has been shown that NO also exhibits in some instances cytotoxic activity against viruses (4) (5), and fungi (6). The production of NO from macrophages is regulated by an inducible enzyme, nitric oxide synthase (iNOS; EC 1.14.13.39) which is capable of producing NO in high amounts and for prolonged periods. It is well established that iNOS is independent of Ca2+ transients and exogenous calmodulin (7), and can be induced by cytokines such as IFN-g and migration inhibitory factor (8). In addition, IFN-g in combination with several agents, including tumor necrosis factor-a (TNF-a) (9), interleukin-1b (IL-1b), interleukin-2 (11) and lipopolysaccharide (LPS) (12) can synergistically promote the NO production by macrophages and some other cells. As such, the induction of iNOS is primarily regulated at the level of gene transcription, although it has been shown that iNOS is up-regulated by alternative pathways, including stabilization of iNOS mRNA (post-transcriptional regulation), enhancement of translation and inhibition of the degradation of NOS protein (7).
Bio-Normalizer (BN) is a natural health food supplement which is made by yeast fermentation of Carica papaya Linn., Pennisetum pupureum Schum. and Sechium edule Swartz. It is produced under strict quality control and no artificial substances are added. Current clinical trials have reported that BN exhibit beneficial therapeutic effect in a variety of human pathologies, including cancer, epilepsy, hepatitis, ulcer, cardiac and renal insufficiency (13). Oral administration of BN to rats has been reported to protect the heart against injury caused by ischemia-reperfusion (14) and decrease the release of monoamine metabolites in iron-induced epileptogenic focus (15). In addition, BN has been shown to have hydroxyl radical scavenging activity using ESR spectroscopy (16). The accumulation of thiobarbituric acid reactive substances and protein carbonyl derivatives were found to be lower in heart homogenates from BN supplemented rats exposed to peroxyl radical generator as compared to non-supplemented ones (14) (17). From such evidence, it has been proposed that the protective effects of BN in various pathologies might be due to its free radical scavenging properties. i) BN has also been reported to upregulate phorbol ester-induced and zymosan-induced superoxide production in rat peritoneal macrophages (18), ii) natural killer cell activity (19), and iii) the level of IFN-g in human blood (20). Such evidence suggests that BN also possesses immuno-modulating activity besides its direct antioxidant activity. However, despite accumulating data on beneficial effects of BN application, little is known about the molecular mechanisms involved in its action. Reactive nitrogen intermediates such as NO, have been suggested to participate in the immune system, as well as other diverse biological activities (2). The present study was designed to investigate whether BN is able to alter the production of NO by macrophages using the mouse macrophage cell line RAW 264.7.
MATERIALS AND METHODS
Reagents
BN was provided by Osato Research Foundation. IFN-g was obtained from Genzyme (Cambridge, MA). Fetal calf serum (FCS) was obtained from the University of California, San Francisco cell culture facility. Oligonucleotide primers were obtained from Clontech (Palo Alto, CA). (6R)-5,6,7,8-tetrahydrobiopterin was obtained from Alexis (San Diego, CA). L-[U-14C] arginine was obtained from DuPont (Wilmington, DE). Other chemicals were purchased from Sigma (St. Louis, MO).
BN solution
BN was suspended in Dulbecco’s phosphate buffer saline (pH 7.4). After incubation for 30 min, the suspension was centrifuged at 15000 x g and the clear supernatant containing the water-soluble BN fraction was used in our experiments.
Cell culture
The murine monocyte/macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection. RAW 264.7 was cultured in 75 cm2 plastic flasks (Flacon, NJ) with Dulbecco’s modifies Eagle’s medium (DMEM) supplemented with 10% FCS and antibiotics. For experiments, macrophages were detached by vigorous pipetting and, after centrifugation, plated in fresh medium. These cells were activated with IFN-g and cultured for 24 h at 37oC in an atmosphere of 5% CO2 plus air.
Deproteinization of sample
The culture medium was deproteinized following the method of Yokoi et al (21). After the macrophages were cultured, the supernatant from the plated cells (400 ml) was mixed with 290 ml of 0.3 M NaOH solution. After incubation for 5 min at room temperature, 290 ml of 5% (w/v) ZnSO4 was added. The mixture was then allowed to stand for another 5 min, and was centrifuged at 2800 x g for 20 min. The supernatant was filtered with a 0.45 mm nylon syringe filter.
Quantification of nitrite and nitrate
Nitrite and nitrate levels in the deproteinized culture supernatant (100 ml) were quantified with Griess reagent using the automated NOx analyzer (Model TCI-NOX 1000), which employs the technique of automated flow injection analysis. Nitrate was determined by reducing it to nitrite using cadmium-copper complex reduction column. For nitrite and nitrate assays, 2.5 x 105 cells were cultured in 24-well plates and treated with appropriate reagents for 24 h before harvesting cell-free supernatants.
Reverse transcription (RT)-Polymerase chain reaction (PCR)
Total RNA was extracted from cultured cells following the method of Chomczynski and Sacchi (22). RNA was reverse transcribed, and the subsequent cDNA was amplified using iNOS and GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) primers. RT-PCR was performed using RNA PCR-Kit and carried out for 35 cycles (1 min at 95oC, 1 min at 65oC, 1.5 min at 72oC). The final PCR reaction products were electrophoresed in 1.7% agarose gel containing 0.2 mg/ml ethidium bromide. The gel was then photographed under ultraviolet transillumination. For quantification, PCR bands on the photograph of the gel were scanned using a laser densitometer linked to a computer analysis system. We normalized the iNOS signal relative to the corresponding GAPDH signal from the same sample, and data were expressed as the iNOS/GAPDH ratio.
NOS activity assay
The enzyme preparation was obtained from RAW 264.7 cells (approximately 3 x 107 cells) activated with 50 U/ml IFN-g and 5 mg/ml LPS for 16~18 h. The cells were collected, washed in DMEM and disrupted by five to six freeze-thaw cycles in 50 mM Tris-HCl (pH 7.4) containing 0.1 mM EDTA, 0.1 mM EGTA, 1 mM phenylmethylsulphonyl fluoride, 1 mM pepstatin A, 2 mM leupeptin and 0.1% 2-mercaptoethanol. The lysate was centrifuged at 15000 x g for 30 min at 4oC, and the supernatant was collected for NOS enzyme activity assay. NOS activity was assayed following [14C]citrulline formation from [14C]arginine, as previously described (23).
RESULTS
Effects of BN on NO production in RAW 264.7 macrophages
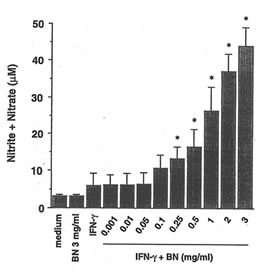
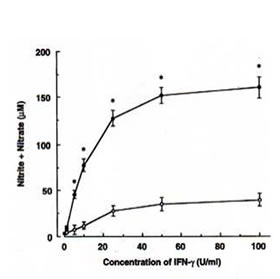
The ability of BN to influence the NO production in RAW 264.7 macrophages was investigated by analysis of accumulated nitrite/nitrate (relatively stable products of reactive nitrogen intermediates) levels in the culture medium as an index for NO synthesis. Unstimulated macrophages produced low levels of NO constitutively. When these resting cells were treated with BN alone, amounts of nitrite/nitrate in the medium were maintained at a level similar to the unstimulated sample. Treated with IFN-g (5 U/ml) increased the production of nitrite/nitrate. A dose-dependent increase in nitrite/nitrate accumulation by BN was observed in macrophages treated with IFN-g (Fig. 1a). When macrophages were cultured with the highest concentration of BN used (3 mg/ml), the IFN-g-induced NO production was approximately 7-fold as compared with that produced by IFN-g treatment alone. In addition, this effect of BN was also observed when macrophages were incubated with various other concentrations of IFN-g (Fig. 1b). IFN-g at 1 U/ml was not effective in producing NO. However, a major increase in NO production was observed at concentrations beyond 5 U/ml of IFN-g. The up-regulation of IFN-g-induced NO production by BN was already significant at 0.25 mg/ml of BN, and reached plateau levels at 3 mg/ml of BN. BN alone or in combination with IFN-g used in this study were not toxic for macrophages as assessed by trypan blue exclusion tests.
Effects of BN on enzyme activity of iNOS
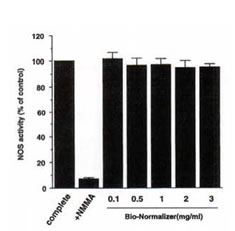
To characterize the mechanism underlying the up-regulation in IFN-g-induced NO production by BN, we examined whether BN directly affected the enzyme activity of iNOS. Figure 2 illustrates the direct effect of BN on iNOS enzyme activity. All values are expressed as a percentage of the control (complete assay mixture). Addition of NG-monomethylarginine (NMMA; 100 mM) which is a competitive inhibitor in NO synthesis, in the complete assay mixture inhibited the iNOS activity by 92%. The experiment was performed by adding various concentrations of BN into the complete assay mixture, however, BN did not affect iNOS activity. This result suggests that the up-regulation of IFN-g-induced NO production by BN is not because of its direct activation of enzyme activity of iNOS.
BN and IFN-g synergistically induce iNOS mRNA expression in RAW 264.7 macrophages
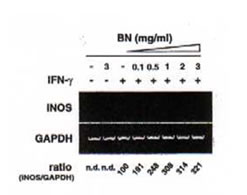
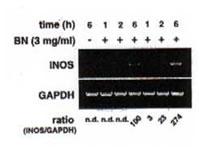
The ability of BN to influence the expression of iNOS mRNA by reverse transcription polymerase chain reaction (RT-PCR) was investigate using specific mouse iNOS primers. Macrophages were incubated with medium, IFN-g (5 U/ml), and BN (3 mg/ml) alone or in combination of IFN-g (5 U/ml) plus BN (at doses ranging from 0.1 to 3 mg/ml). The total RNA was extracted 6 h after stimulation. Macrophages did not express iNOS mRNA either constitutively or after treatment with BN alone (Fig. 3a). However, low levels of iNOS mRNA were detectable following treatment of macrophages with IFN-g alone. The combination of IFN-g and BN (1 mg/ml) caused an augmentation in iNOS mRNA expression of approximately 3-fold over that induced by IFN-g alone. The synergistic interaction between IFN-g and BN in the induction of iNOS mRNA reached a plateau level at a dose of 3 mg/ml BN. Furthermore, to determine the time dependent effect of BN, macrophages were incubated for various periods in the presence or absence of IFN-g. The IFN-g dependent induction in iNOS mRNA was not observed even 2 h after stimulation (Fig. 3b). In contrast, addition of 3 mg/ml BN caused a major increased of IFN-g-induced iNOS mRNA expression that was already detectable after 2 h of treatment. After 6 h of incubation, BN caused about 3-fold augmentation of iNOS mRNA expression over that induced by IFN-g alone, similar to that observed in Fig. 3a. The augmented expression of iNOS mRNA by IFN-g alone or IFN-g plus BN was associated with a parallel production of NO, as measured by accumulation of nitrite/nitrate in culture supernatants. These data demonstrate that BN acted synergistically with IFN-g in a dose and time dependent fashion for the induction of NOS mRNA expression in macrophages.
Effect of BN on the half-life of iNOS mRNA
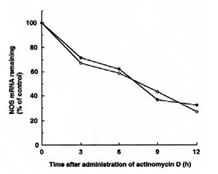
Experiments were performed to investigate whether BN could influence the stability of iNOS mRNA. We compared the half-life of IFN-g plus BN induced iNOS mRNA with that of cells treated with IFN-g alone. Macrophages were incubated with IFN-g (5 U/ml) alone or with IFN-g plus BN (3 mg/ml) for 6 h before addition of actinomycin D (5 mg/ml) an inhibitor of RNA synthesis, and then the total SNA was harvested at different time points (ranging from 0 to 12 h). The half-life of iNOS mRNA in macrophages treated with IFN-g plus BN was approximately 7 h, and it was not significantly different from that of IFN-g alone treated cells (Fig. 4). This result suggests that the mechanism by which BN augments iNOS mRNA expression is not due to the increase of stability of iNOS mRNA.
Effect of BN on the induction of TNF-a, IL-1b mRNA expression in IFN-g-treated RAW 264.7 macrophages
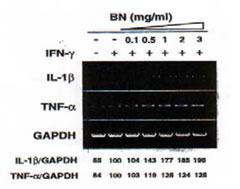
We investigated whether BN could induce the TNF-a or IL-1b genes that are implicated in immune system (Fig. 5). These mRNA were constitutively detectable in untreated macrophages, and the expressions of these genes were slightly enhanced by treatment with IFN-g (5 U/ml). BN caused a further increase in TNF-a, IL-1b mRNA expression approximately 1.2-fold and 2-fold respectively compared to that induced by IFN-g alone. These results suggest that BN together with iNOS mRNA can upregulate other immune response mediator genes such as TNF-a and IL-1b.
DISCUSSION
Bio-Normalizer was found to influence NO production in RAW 264.7 macrophages synergistically with IFN-g by enhancing the accumulation of iNOS mRNA without any alteration in the stability of mRNA. Furthermore, it was observed that BN also slightly increased the expression of TNF-a or IL-1b mRNA in IFN-g-treated macrophages. Despite the ability of BN to induce together with IFN-g an activation of the cellular response in these cells, no effect on cell proliferation (data not shown) or on NO production was detected when cells were incubated with BN alone, even at 3 mg/ml. This observation strongly suggests that BN itself does not exhibit any toxic affects under our experimental conditions and that BN does not contain endotoxic substances such as LPS that can induce iNOS activity. This evidence suggests that BN acts as a modulator of cellular function, rather than direct stimulator of NO production in macrophages. The present study does not provide direct evidence on the mechanism underlying this gene induction effect of BN. However, it is likely that BN somehow modulates IFN-g induction of iNOS, by up-regulating the rate of transcription. This could occur either by an increase in the rate of translation or by prevention of degradation of the iNOS protein. Recent studies have shown that cAMP can augment IFN-g-induced NO production (24) (25). The mechanism by which BN enhances NO production possibly mimics that caused by increment of the cAMP level. This is because the treatment of cAMP plus IFN-g caused accumulation of iNOS mRNA without any change in its stability, cAMP itself was shown to be not sufficient to enhance NO production (25). A major component of BN is a mixture of plant and yeast glycopolysaccharides, amino acids (tryptophan, leucine, glutamic and aspartic acids), vitamin B6, vitamin C and glucose. Further experiments are needed to define the substances in BN that act with IFN-g on iNOS gene expression or regulate the intracellular level of second messengers, including cAMP.
It is known that NO plays a multifaced role in various physiological conditions and immune responses (1) (2). NO produced by iNOS is thought to be potentially toxic and much attention has been given to understanding its cytotoxic effects, especially on pathogenic bacteria and tumor cells (2). NO is also involved in immunosuppression through inhibition of host the T-lymphocyte response (26). However, whether this is a critical function to the host is not yet established. This may be because NO exerts a feedback inhibition of its own production through its anti-proliferative effect on T-lymphocytes and thus NO can control the potential for hyper-reactivity. Alternatively, there is evidence that tumor regression is directly correlated with iNOS expression within the tumor lesion (27). Moreover, it has been reported that activation of iNOS might be a potential therapeutic target for the treatment of cancer metastasis by causing vasodilatation (28), inhibition of platelet aggregation (29), inhibition of angiogenesis (30), or induction of apoptosis in the cells expressing iNOS (31). Also, there is a report that suggests activation of iNOS may be effective in allergic disorders (32).
NO has been reported to decrease tissue injury during pathological events associated with excess reactive oxygen species formation. NO has also been shown to exhibit antioxidant activity by inhibiting lipid peroxidation (33) (34) (35) and cellular oxidative damage produced by tert-butyl-hydroperoxide (36). The antioxidant effects of NO are due to its direct interactions with alkoxyl or peroxyl radicals formed during lipid peroxidation. Rubbo, et. al. reported that NO is dependent on the quantity and the source of its formation, and whether its activities differ at the sites where effector cells exist. The synthesis of NO in human macrophages still remains controversial. However, there is indirect evidence of its synthesis in monocyte-derived macrophages (38), alveolar macrophages (39) and neutrophils (40). Therefore, the findings of this study concerning efficacy of BN on NO production using murine macrophages, a cell line where the mechanisms of NOS expression are well-defined, are likely to be relevant to human leukocytes.
Our laboratory previously has reported antioxidant effects of BN supplementation against oxidative damage to the rat heart caused by ischemia-reperfusion injury or by chemically-induced peroxyl radicals (14). In present study, it is suggested that BN might modulate cellular metabolism and physiological functions upon induction of the iNOS gene. Normally, nitrogen dioxide (NO2.) and peroxynitrite (ONOO-) are highly toxic in excess and may be effective in host defense against microbial infection and tumors. However, it is thought that the balance between NO and some other free radical species is important (37). Moreover, exposure of NO to hepatocytes has been shown to prevent TNF-a-induced cytotoxicity (41). NO may also lead to the generation of potentially cytoprotective species such as nitrosoglutathione which can induce coronary vasodilation and improve the functional recovery of ischemic hearts (42). Also nitrosoglutathione protects against hydrogen peroxide-mediated cytotoxocity in lung fibroblasts (43). The findings reported in this study might be one of the properties of BN that may explain its beneficial effects in various disorders that have been reported so far and expand the possibilities that BN acts as a multifunctional agent.
Acknowledgements
Supported by National Institutes of Health (GM 27345)
Figure legend
Fig. 1. BN synergizes with IFN-g for NO production in RAW 264.7 macrophages.
- Dose-dependent effect of BN on NO production. Macrophages were incubated with medium alone, IFN-g (5 U/ml) alone or with IFN-g in the presence of the indicated concentrations of BN for 24 h. All values represent the mean ± SD of three independent experiments. *p<0.05 compared with that of IFN-g alone treatment.
- Efficacy of IFN-g on NO production by BN. Macrophages were incubated with the indicated concentrations of IFN-g in either the absence (m) or the presence (l) of 3 mg/ml of BN for 24 h. All values represent the mean ± SD of three independent experiments. *p<0.05 compared with that of IFN-g alone treatment.
Fig. 2. BN does not affect iNOS enzyme activity. NOS activity was determined by the conversion of radiolabeled arginine to citrulline using a cytosolic preparation from macrophages. Complete (as control) indicates that all constituents listed in Methods section are contained in the reaction mixture. NG-monomethylarginine (NMMA) was present in the assay at 100 mM. BN was added into the complete assay mixture as indicated. All values are expressed as a percentage of the control (100% : 178 ± 3 pmol/mg protein/min) and represent the mean ± SD of three independent experiments.
Fig. 3. Synergistic induction of iNOS mRNA expression by IFN-g plus BN.
- Dose-dependent induction. Macrophages were incubated with medium alone, BN (3 mg/ml) alone, IFN-g (5 U/ml) alone or with IFN-g in the presence of the indicated concentration of BN for 6 h, and total cellular RNA was applied to RT-PCR using iNOS specific primer. All values are expressed as a percentage of the control (IFN-g alone) for iNOS/GAPDH ratio from results obtained by RT-PCR.
- Time-course induction. Macrophages were incubated with IFN-g (5 U/ml) alone or with IFN-g plus BN (3 mg/ml) for various periods as indicated, and total cellular RNA was applied to RT-PCR. All values are expressed as a percentage of the control (IFN-g alone, 6 h) for iNOS/GAPDH ratio from results obtained by RT-PCR.
Fig. 4. Effect of BN on iNOS mRNA stability in IFN-g-treated RAW 264.7 macrophages. Macrophages were incubated with IFN-g (5 U/ml) alone (m) or with IFN-g plus BN (3 mg/ml) (l) for 6 h. Then, actinomycin D (5 mg/ml) was added to the culture, and total cellular RNA was extracted at the indicated time and applied to RT-PCR. Data are presented as the relative amount of iNOS mRNA remaining after addition of actinomycin D and normalization to the respective amount of GAPDH mRNA.
Fig. 5. Dose-dependent induction of IL-1b, TNF-a mRNA by IFN-g plus BN. Macrophages were incubated with medium alone, IFN-g (5 U/ml) alone or with IFN-g in the presence of the indicated concentrations of BN for 6 h, then total cellular RNA was extracted and applied to RT-PCR using IL-1b or TNF-a specific primers. All values are expressed as a percentage of the control (IFN-g alone) from results obtained by RT-PCR.
REFERENCES
- Moncada, S., Palmer, R.M., and Higgs, E.A. (1991) Pharmacol. Rev. 43, 109-142.
- Nathan, C. (1992) Faseb J. 6, 3051-3064.
- Knowles, R.G., and Moncada, S. (1992) Trends Biochem. Sci. 17, 399-402.
- Karupiah, G., and Harris, N. (1995) J. Exp. Med. 181, 2171-2179.
- Karupiah, G., Xie, Q.W., Buller, R.M., Nathan, C., Duarte, C., and MacMiking, J.D. (1993) Science. 261, 1445-1448.
- Nathan, C.F., and Hibbs, J.B., Jr. (1991) Curr. Opin. Immunol. 3, 65-70.
- Nathan, C.F., and Xie, Q.W. (1994) J. Biol. Chem. 269, 13725-13728.
- Cunha, F.Q., Weiser, W.Y., David, J. R., Moss, D.W., Moncada, S., and Liew, F.Y. (1993) J. Immunol. 150, 1908-1912.
- Drapier, J.C., Wietzerbin, J., and Hibbs, J.B., Jr. (1988) Eur. J. Immunol. 18, 1587-1592.
- Stuehr, D.J., Cho, H.J., Kwon, N.S., Weise, M.F., and Nathan, C.F. (1991) Proc. Natl. Acad. Sci. USA. 88, 7773-7777.
- Cox, G.W., Melillo, G., Chattopadhyay, U., Mullet, D., Fertel, R.H., and Veresio, L. (1992) J. Immunol. 149, 3290-3296.
- Stuehr, D.J., and Marletta, M.A. (1987) J. Immunol. 139, 518-525.
- Ichifuji, O. (1996) in Tainai-kakumei (F, K., Ed.), 1.
- Haramaki, N., Marcocci, L., D’ Anna, R., Yan, L.J., Kobuchi, H., and Packer, L. (1995) Biochem. Mol. Biol. Int. 36, 1263-1268.
- Santiago, L.A., Osato, J.A., Kabuto, H., and Mori, A. (1992) Med. Sci. Res. 21, 139-141.
- Santiago, L.A., Osato, J.A., Hiramatsu, M., Edamatsu, R., and Mori, A. (1991) Free Radic. Biol. Med. 11, 379-383.
- Marcocci, L., D’ Anna, R., Yan, L.J., Haramaki, N., Kobuchi, H., and Packer, L. (1995) Biochem. Mol. Biol. Int. 36, 1263-1268.
- Osato, J.A., Korkina, L.G., Santiago, L.A., and Afanas’ev, I.B. (1995) Nutrition 11 (5 Suppl). 568-572.
- Okuda, H., Ominami, H., Zhou, A., Osato, A., and Santiago, L.A. (1993) The Clin. Report. 27, 4249-4258.
- Santiago, L.A., Uno, K., Kishida, T., Miyagawa, F., Osato, J.A. and Mori, A. (1994) Neurosciences. 20, 149-152.
- Yokoi, I., Habu, H., Kabuto, H., and Mori, A. (1996) in Methods in Enzymology (Packer, L., Eds.) Vol. 268, pp.152-159.
- Chomczynski, P. and Sacchi, N. (1987) Anal. Biochem. 162, 156-159.
- Kobuchi, H., Droy-Lefaix, M.T., Christen, Y., and Packer, L. (1997) Biochem. Pharmacol. 53, 897-903.
- Alonso, A., Carvalho, J., Alonso-Torre, S.R., Nunez, L., Bosca, L., and Sanchez Crespo, M. (1995) J. Immunol. 154, 6475-6483.
- Mullet, D., Fertel, R.H., Kniss, D., and Cox, G.W. (1997) J. Immunol. 158, 897-904.
- Tomioka, H., and Saito, H. (1992) J. Leukoc. Biol. 51, 24-31.
- Xie, K., Huang, S., Dong, Z., Gutman, M. and Fidler, I.J. (1995) Cancer Res. 55, 3123-3131.
- Palmer, R.M., Ferrige, A.G. and Moncada, S. (1987) Nature. 327, 524-526.
- Radomski, M.W., Palmer, R.M. and Moncada, S. (1990) Br. J. Pharmacol. 101, 325-328.
- Pipili-Synetos, E., Sakkoula, E., Haralabopoulos, G., Andriopoulou, P., Peristeris, P., and Maragoudakis, M.E. (1994) Br. J. Pharmacol. 111, 894-902.
- Xie, K., Huan S., Dong, Z., Juan,S.H., Gutman, M., Xie, Q.W., Nathan C., and Fidler, I.J. (1995) J. Exp. Med. 181, 1333-1343.
- Heyman, S.N., Karmeli, F., Brezis, M., and Rachmilewitz, D. (1997) Br. J. Pharmacol. 120, 1545-1551.
- Wink, D.A., Hanbauer, I., Khrisna, M.C., DeGraff, W., Gamson, J., and Mitchell, J.B. (1993) Proc. Natl. Acad. Sci. USA. 90, 9813-9817.
- Hogg, N., Kalyanaraman, B., Joseph, J., Struck, A., and Parthasarathy, S. (1993) FEBS Lett. 334, 170-174.
- Hayashi, K., Noguchi, N., and Niki, E. (1995) FEBS Lett. 370, 37-40.
- Gorbunov, N., Yalowich, J.C., Gaddam, A., Thampatty, P., Ritov, V.B., Kisin, E.R., Elsayed, N.M. and V.E. Kagan (1997) J. Biol. Chem. 272,12328-12341.
- Rubbo, H., Radi, R., Trujillo, M., Telleri, R., Kalyanaraman, B., Barnes, S., Kirk, M., and Freeman, B.A. (1994) J. Biol. Chem. 269, 26066-26075.
- Denis, M., (1991) J. Leukoc. Biol. 49, 380-387.
- Sherman, M.P., Loro, M.L., Wong, V.Z., and Tashkin, D.P. (1991) J. Protozool. 38, 234S-236S.
- Wheeler, M.A., Smith, S.D., Garcia-Cardena, G., Nathan, C.F., Weiss, R.M., and Sessa, W.C. (1997) J. Clin. Invest. 99, 110-116.
- Kim, Y.M., de Vera, M.E., Watkins, S.C., and Billiar, T.R. (1997) J. Biol. Chem. 272, 1402-1411.
- Konorev, D.A., Tarpey, M.M., Joseph, J., Backer, J.E., and Kalyanaraman, B. (1995) J. Pharmacol. Exp. Ther. 274, 200-206.
- Wink, D.A., Cook, J.A., Pacelli, R., DeGraff, W., Gamson, J., Liebmann, J., Krishna, M.C., and Mitchell, J.B. (1996) Arch. Biochem. Biophys. 331, 241-248.
FIG. 1 A
FIG. 1B
Fig. 2
Fig. 3a
Fig. 3b
Fig. 4
Fig. 5