| Title | Ferric Nitrilotriacetate Induced DNA and Protein Damage: Inhibitory Effect of a Fermented Papaya Preparation |
|---|---|
| Year | 2000 |
| Author | GERALD RIMBACH, QIONG GUO, TAKASHI AKIYAMA, SEIICHI MATSUGO, HADI MOINI, FABIO VIRGILI, and LESTER PACKER |
| Publisher | Anti-Cancer Research |
Ferric Nitrilotriacetate Induced DNA and Protein Damage: Inhibitory Effect of a Fermented Papaya Preparation
GERALD RIMBACH1, QIONG GUO1, TAKASHI AKIYAMA1, SEIICHI MATSUGO2, HADI MOINI1, FABIO VIRGILI3, and LESTER PACKER1
1Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720-3200, USA.; 2Department of Applied Chemistry and Biotechnology, Faculty of Engineering, Yamanashi University, 4-3-11 Takeda, Kofu 400-8511, Japan; 3National institute of Nutrition, Via Ardeatina 546, Rome, Italy
Abstract. The carcinogen Fe-NTA catalyzes the hydrogen peroxide-derived production of free radicals and possibly acts through a mechanism involving oxidative stress. Fermented papaya preparation (FPP) has been reported as a natural antioxidant able to prevent lipid peroxidation in vitro and in vivo. However, little is known about the antioxidant properties of FPP regarding iron-mediated oxidative damage to DNA and proteins. In the present study FPP protected supercoiled plasmid DNA against Fe-NTA plus H2O2 induced single and double strand breaks. Similar protective effects of FPP were evident when human T-Lymphocytes were challenged with FeNTA/H2O2 and DNA damage was determined using the Comet assay. Fe-NTA/ H2O2 also induced fragmentation of bovine serum albumin (BSA) in vitro and depleted cellular GSH Level in lymphocytes. BSA fragmentation and GSH depletion were dose-dependently counteracted by FPP. EPR spin trapping studies demonstrated that antioxidant properties of FPP are related to both hydroxyl scavenging as well as iron chelating properties.
Redox cycling is a characteristic of transition metals including iron, which is centrally involved in the generation of reactive oxygen species. Redox reactive iron is generally present in a weakly chelated form (1). Nitrilotriacetic acid (NTA) is a synthetic aminotricarboxlic acid, which forms water-soluble complexes with iron at neutral pH. NTA is a constituent of various domestic and hospital detergents and is a common water contaminant (2). A high incidence of renal cell carcinoma is prevalent in mice following repeated intraperitoneal Fe-NTA injections (3). The carcinogenity of Fe-NTA seems to be associated with the interaction of NTA with Fe3+, since no tumor formation was observed by administration of NTA alone (3). Fe-NTA has also been reported to induce severe hemochromatosis, diabetes as well as adenomocarcinoma in laboratory
Correspondence to: Dr. Lester Packer, Department of Molecular and Cell Biology, 251 Life Science Addition, University of California, Berkeley, CA 94720-3200, U.S.A., Fax: 1-510-642-
8313, Tel: 1-510-642-1872, e-mail: Packer@socrates.berkeley.edu
Key Words: Ferric nitrilotriacetate, free radicals, DNA and protein damage, glutathione, fermented papaya preparation, Jurkat cells.
0250-7005/2000 $2.00 + .40
animals (4). It is that the mechanism of Fe-NTA toxicity is mediated via the production of free radicals which in turn induce oxidative damage to lipids, proteins and DNA. Additionally, other reports have shown that Fe-NTA increases both hepatic ornithine decarboxylase activity and 3H thymidine incorporation possibly through the generation of oxidative stress (5, 6). It has been previously shown that Fe-NTA decreased antioxidant enzyme activities with the concomitant increase in the production of lipid peroxides and hydrogen peroxide (7). Antioxidants such as vitamin E and butylated hydroxyanisol, as well as the iron chelator desferrioxamine have been reported to partially prevent the toxic effect of Fe-NTA (8, 9). There is a growing interest in the utilization of plant extracts as dietary food supplements. A wide spectrum of beneficial activity for the human health has been advocated for such supplements due, at least in part to their antioxidant activity (10). More recently the ability of antioxidant nutrients to affect cell response and gene expression has been reported in vitro, providing a novel and different mechanistic perspective underlying the biological activity of plant derived nutraceuticals (11, 12, 13). Fermented papaya preparation (FPP) is made by yeast fermentation of Carica papaya Linn., Pennisetum purpeum Schum. and Sechium edule Swartz and is used as a natural food health supplement in different parts of the world. FPP has been shown to upregulate phorbol ester or zymosan-induced superoxide production in rat peritoneal macrophages (14), natural killer cell activity (15), and the level of IFN-γ in human blood (16). Recent studies of our laboratory demonstrated that FPP affects NO and hydrogen peroxide production as well as tumor necrosis factor alpha secretion in RAW 264.7 macrophages (17). Such evidence suggests a role of FPP as an immunomodulator. It has also been reported that FPP protects the brain of aged rodents in vivo challenged either by oxidative stress (18) or by ischemiareperfusion injury (19). Furthermore, the accumulation of thiobarbituric acid reactive substances were found to be lower in heart homogenates from FPP supplemented rats exposed to peroxyl radicals as compared to non-supplemented controls (20). From these reports it has been proposed that beside immuno-modulating FPP possess also antioxidant activities. However, the underlying mechanisms by which FFP acts as an antioxidant are largely unknown. In the present study both hydroxyl radical scavenging and iron chelating properties of FPP have been evaluated. Furthermore, the ability of FPP to combat Fe-NTA oxidative damage to DNA and proteins has been investigated both in vitro as well as in cultured cells.
Materials and Methods
Cell culture. Jurkat cells, a human acute leukemic T-cellline (clone E6-1, American Type Culture Collection, ATCC, Manassas, VA) were grown in RPMI 1640 medium (Gibco BRL, Gaithersburg, MD) supplemented with 10 % FCS, 100 U/ml penicillin and 100 µg/ml streptomycin, 110 mg/ml sodium pyruvate and 2 mM L-glutamine (Cell culture facility, University of California, San Francisco). Cell were maintained in a standard culture incubator with humidified air containing 5% CO2 at 37°C. FPP (Bionormalizer™, Osato Research Foundation, Japan) was dissolved in PBS (pH 7.4) and added to the Jurkat culture medium at the
indicated concentrations.
Preparation of Fe3+-NTA solution. Fe-NTA solution was prepared according to the method of Awai et al (21). Briefly, a solution of ferric nitrate dissolved in 1.0 N HCl was mixed with a 4-fold molar excess of NTA and the pH was adjusted to 7.4. using sodium bicarbonate.
Single and double DNA strand breaks. The reaction mixture (15 µl final volume, adjusted with distilled water) for assaying Fe-NTA plus H2O2 induced plasmid DNA damage contained 0.1 µg superhelical pUC18 DNA, 100 µM Fe-NTA and 100µM H2O2 with and without various concentrations of FPP, in phosphate buffered saline, pH 7.4. The reaction was performed at 37°C for 30 min, and stopped by adding 10 mM desferrioxamine. After addition of 3.0 µl 6-fold concentrated gel loading buffer to the reaction mixture, the sample was applied to a slot of a 0.8% agarose gel containing 0.5 µg/ml ethidium bromide, and electrophoresed for conformational analysis of DNA.
Comet assay. The reaction mixture (1 ml final volume, adjusted with PBS) containing 1×105 Jurkat cells, 50 µM Fe-NTA, 50 µM H2O2 and various concentrations of FPP was incubated at 37°C for 60 min. The reaction was stopped by chilling the samples to 0°C and centrifugation at 5,000 g for 5 min. Comet assay formation was determined with a test kit (Trevigen, Inc., MD) according to the manufactors protocol. Comets were evaluated by fluorescence microscopy. Cell were graded according to their DNA damage into 5 classes from undamaged (class 0), to maximally damaged, (class 4). A total damage score for the slide was derived by multiplying the number of cells assigned to each grade of damage by the numeric value of the grade and summing over all grades. Thus total score or 100 randomly selected cells could range from 0 (no damage) to 400 (corresponding to 100 cells of damage grade 4).
Assay for protein fragementation. The ability of FPP to inhibit protein fragmentation was tested by incubation 1 mg/ml bovine serum albumin (Sigma) with 50 µM Fe-NTA, in phosphate buffer (pH 7.4) in the presence of increasing concentration of FPP. Reaction was started by the addition of hydrogen peroxide (2.5 mM final concentration). After 24 hours of incubation at 37°C, portions of reaction mixtures were loaded on a 10 % SDS-PAGE according to Hunt et al. (22). Protein fragments were detected by staining with Coomassie brilliant blue.
HPLC determination of glutathione. Cells were pelleted (125 x g for 5 min) and deproteinized by treatment with 5% monochloroacetic acid. Following the acid treatment, the mixtures were snap-frozen in liquid nitrogen at -80°C for the HPLC determination of GSH. Samples were eluted form an Altima C18 250 mm, 4.6 I.D., 5 µM column (Alltech, Deerfield, IL) with 100 mM monochloroacetic acid (pH 3.0) containing 5% methanol and detected with a thiol-selective gold-mercury column (23).
Electron paramagnetic resonance spectroscopy spin trapping measurements.
The antiradical activity of FPP was measured by the diphenyl-p-picrylhydrazyl (DPPH) assay as described previously (24). 50 µM DPPH (Sigma Chemical Co., St. Louis, MO) was mixed with the indicated concentrations of FPP dissolved in phosphate buffer (50 mM, pH= 7.4). Hydroxyl radical scavenging activity of FPP was evaluated both in an iron independent (NP-III/UV) as well as in iron-dependent systems (PeNTA plus H2O2, Fenton reaction). For the iron independent system 20 mM NP-III (N,N-bis(2-hydroperoxy-2-methoxyethyl-1,4,5,8, naphthalene-tetracaboxylic diimide) were irradiated by an UV lamp at 365nm for 1 min according to the procedure of Matsugo and co-workers (25). The reaction mixture for the Fe-NTA/H2O2 system contained 10 µM Fe-NTA and 80 µM H2O2. The reaction mixture for the Fenton systems contained 25 µM FeSO4 and 50 µM H2O2. In all three systems the spin trap 5,5 dimethyl-1-pyrroline-N-oxide (DMPO) dissolved in phosphate buffer was as used at a concentration of 200 mM. EPR spectra were recorded using an IBM ER 200D-SRC EPR spectroscopy (Danbury, CT). EPR spectrometer settings were as follows: central field 3475 G; modulation frequency 100 kHz; modulation amplitude 2.0 G; microwave power 10mW; scan width 200 G, gain 6.3 x 105; temperature 298K.
Nuclear magnetic resonance spectroscopy. The 1H and 13C NMR spectra of FPP were recorded at room temperature on a Brucker Avance 400 ICON NMR spectrometer (1H: 500 MHz, 13C:100 MHz). FPP (10 mg) was dissolved in 700 µl of D2O and transferred to 5 mm phai NMR tube. Accumulation was carried out 16 times for 1H and 1024 times for 13C respectively.
Data presentation. Data in figures are the mean ± standard deviation (SD) of three different experiments performed in triplicate. One representative picture out of at least three is present regarding DNA single and double strand breaks, comet assay and BSA fragmentation.
Results
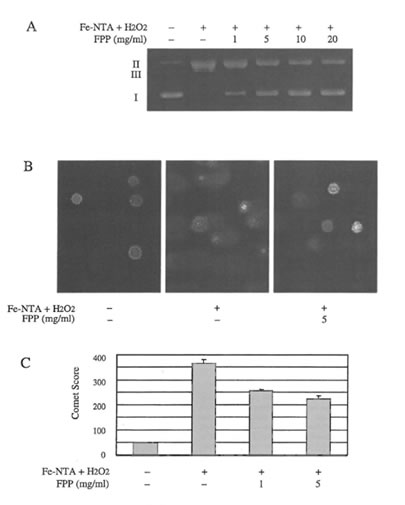
DNA damage in vitro and in T-lymphocytes. Untreated pUC18 DNA was composed of abundant syperhelical DNA (form I) and small amounts of nicked open circular DNA (form II). No linear form DNA (form III) was present. Plasmid DNA remained intact after incubation either with Fe-NTA, H2O2 or FFP alone. (data not shown). The treatment of pUC18 DNA with a mixture of Fe-NTA and H2O2 increased nicked open circular DNA with single strand breaks as well as the linear form with double strand breaks significantly. However FPP dose-dependently inhibited Fe-NTA plus H2O2 induced DNA damage (Figure 1A).
The effect of FPP on Fe-NTA plus H2O2 induced DNA damage was also investigated in a cellular system using the comet assay. In agreement with the plasmid DNA data, exposure of human T-lymphocytes to Fe-NTA plus H2O2 induced a clear DNA damage as indicated by the comet formation (Figure 1B). Pretreatment of Jurkat cells with FPP decreased Fe-NTA plus H2O2 induced DNA damage as shown in Figure lB. The corresponding DNA damage scores are given in Figure 1 C.
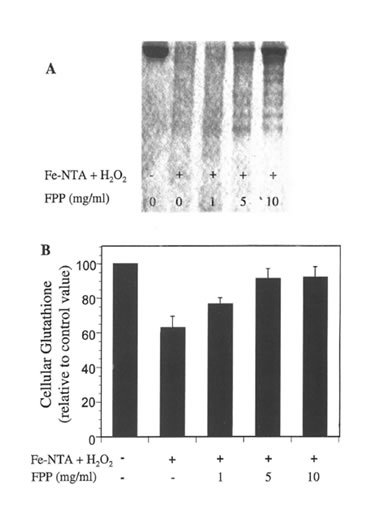
Protein fragmentation in vitro and cellular GSH levels. Representative SDS-PAGE of BSA samples treated with Fe-NTA plus H2O2 in the absence and presence of FPP are shown in Figure 2A. Hydrogen peroxide or Fe-NTA alone resulted in no apparent change in the original BSA band (data not shown) However, in the presence of Fe-NTA plus H2O2 a marked fragmentation of the original BSA band was evident. FPP, at the highest concentration tested, partially attenuated Fe-NTA/H2O2 induced BSA damage.
Treatment of Jurkat cells with Fe-NTA plus H2O2 significantly depleted cellular GSH levels to about 40% as compared to untreated control cells (absolute GSH concentration= 2.20 nmol/106 cells). However, the supplementation of human T-lymphocytes with increasing concentrations of FPP dose-dependently counteracted Fe-NTA plus H2O2 induced GSH depletion (Figure 2B).
Figure 1. A) Effect of increasing concentrations of FPP on DNA single and double strand breaks of superhelical pUC18 plasmid DNA induced by FeNTA/H2O2; B) Representative microphotographs of microgel electrophoresed T-lymphocytes treated without and with Fe-NTA/H2O2 in the absence and presence of FPP, DNA damage was measured with the comet assay; C) Relative score of damaged DNA in T-lymplzocytes induced by Fe-NTA/ H2O2 in the absence and presence of FPP as measured with the comet assay.
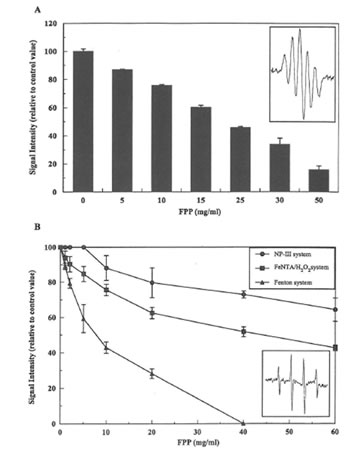
Radical scavenging activity of FPP. First, the ability of FPP to scavenge DPPH radicals was investigated. DPPH is a stable free radical (inset of Figure 3A) which can be detected by EPR. As shown in Figure 3A, DPPH radical formation was dose-dependently inhibited by FPP. A concentration as high as 25 mg/ml resulted in a 50% (IC50) inhibition of the DPHH signal.
In the next step hydroxyl radical scavenging activity of FPP has been studied in both iron independent (NP-III/UV) as well as iron-dependent systems (Fe-NTA H2O2, Fenton) in order to discriminate between antioxidant and iron chelating properties of FPP. The typical EPR spectrum of the DMPOOH spin adduct was detected in all three systems used (inset of Figure 3B). Concentrations of FPP scavenging 50% of the hydroxyl radical were 8.0 mg/ml (Fenton) and 45.0 mg/mL (Fe-NTA plus H2O2) respectively. However, in the NPIII/UV system the highest concentration of FPP (60 mg/ml) resulted only in a 30% inhibition of hydroxyl formation.
Discussion
A large number of dietary factors afford protection against carcinogensis. Although the exact underlying mechanisms are not completely elucidated, many of the beneficial properties of chemopreventive molecules have been attributed to their ability to upregulate carcinogen metabolizing enzymes and/or to bind to toxicants. Alternatively, various constituents of the human diet act as antioxidants thereby counteracting the increased formation of free radicals generated by the toxicants (7). In this regard, the term nutraceutical has been recently established in order to describe nutrients able to significantly and positively affect human health (26).
In the present study Fe-NTA caused marked elevations of oxidatively damaged DNA and protein both in vitro as well as in human T-lymphocytes. It is believed that the toxicity of NTA is due to the binding of Fe3+ to form complexes, which in turn generate free radicals. The nature of reactive oxygen species, which induced DNA and protein modifications, was studied by EPR using DMPO as a spin trap. In accordance to previous finding by Ogino and Okada (1) no detectable EPR signal was evident in the absence of H202 (data not shown). However, in the presence of H2O2, Fe-NTA showed a typical four line spectrum with a 1:2:2:1 hyperfine splitting. Its hyperfine splitting constants (aN = aH= 14.9 G) are the same as reported in the literature for the DMPO-OH spin adduct (27), suggesting that hydroxyl radicals are possible candidates for the Fe-NTA induced damage in the presence of H2O2. Furthermore, in this regard it has been demonstrated that hydroxyl radical scavengers such as DMSO, D-mannitol and ethanol (4) significantly counteracted oxidative damage due to Fe-NTA/H2O2 in vitro as well as in cultured cells. These findings in conjunction with the present results strongly support the hypothesis that DNA damage was mainly caused by hydroxyl radicals, generated by the reaction of Fe-NTA with H2O2. Protein molecules also can be targets f reactive oxygen species. Metal catalyzed oxidation of proteins is supposed to be a site-specific reaction and iron-binding sites have been postulated on protein molecules, where the metal catalyzed protein damage is likely to occur (28). So far little is known about interactions between Fe-NTA and proteins. Under the conditions investigated Fe-NTA plus H2O2 caused significant BSA fragmentation. In a previous investigation it has been shown that Fe-NTA plus H2O2 caused bi-tyrosine formation and tryptophan decrease of BSA, suggesting that this damage is mainly related to free radical formation (1). Since cytosolic peptides are candidates for iron mediated damage the effect of Fe-NTA plus H2O2 on GSH levels in Tlymphocytes has also been investigated. Fe-NTA plus H2O2 significantly depleted levels of GSH in Jurkat cells to about 40% possibly due to an increased
Figure 2. A) Representative SDS-PAGE of BSA samples treated witlr Fe-NTA/H2O2 in the absence and presence of increasing concentrations of FPP; B) Effects of FPP and Fe-NTA/ H2O2 on glutathione levels in humas T-lymphocytes.
Figure 3. A) DPPH radical scavenging activity of FPP; B) Effect of increasing concentrations of FPP on DMPO-OH spin adducts formed by iron independent as well as iron dependent hydroxyl radical generating systems.
formation of oxidized glutathione. In addition to the present findings in Jurkat cells, it has been demonstrated that when injected to laboratory rats Fe-NTA lead a dose-dependent decrease in glutathione reductase and glutathione-S-transferase (29), enzymes, which are important for GSH synthesis. In contrast, γ-glutamyl transpeptidase activity was significantly increased by Fe-NTA. This enzyme catalyzes the degradation of GSH, which may lead to a higher accumulation of cysteinyl, glycine and cysteine. Elevated levels of these GSH metabolites have been suggested to enhance reduction of Fe-NTA to its ferrous complex, which in turn enhances oxidative cellular damage (30). Overall, present data as well as reports from the literature suggest that the depletion in cellular GSH levels induced by Fe-NTA H2O2 might be related to both direct oxidation of GSH, to GSSG as well as modulation of key enzymes centrally involved in GSH homeostasis and turnover.
In the current study it has been clearly shown that FPP protected both in vitro as well as in human T-lymphocytes against Fe-NTA/ H2O2 induced DNA and protein damage. The underlying mechanisms by which FFP combats oxidative stress in biological systems are not completely understood. Antioxidant action can be exerted through different mechanisms, e.g. metal chelation, radical scavenging as well as electron donation (31). In order to get further insights into FPP’s mode of action it was desirable to generate hydroxyl radicals both with iron dependent as well iron independent systems. In the present investigation the photosensitive organic hydroperoxide NP-III has been used to generate hydroxyl radicals in the absence of iron. NP-III is a novel compound developed by our laboratory that generates hydroxyl radicals upon irradiation with UV under controllable experimental conditions (32). In combination with the Fenton reaction it is thereby possible to distinguish between metal chelating as well as radical scavenging activities of compounds of interest. Under the conditions investigated, FPP acted as an antioxidant due to both hydroxyl scavenging as well as iron chelating properties. By comparing hydroxyl radical scavenging activity of FPP between the Fenton and NP-III system (Figure 3) it can be concluded that iron chelating is an important mechanism, which significantly contributes to the antioxidant mode of action of FPP. Iron binding affinity of FPP might prevent that Fe3+ is being reduced back to Fe2+, which in turn promotes the formation of hydroxyl radicals via Fenton reaction. Removal of Fe3+ by FPP would thus further reduce hydroxyl radical generation (33). However it should be mentioned that relatively high concentrations of FPP were necessary to combat Fe-NTA induced oxidative damage. Moreover, it has not been clarified yet which particular constituents of FFP are mainly responsible for its antioxidant acitivity. NMR spectroscopy indicated that FPP contained no aromatic protons or carbons (data not shown) thus antioxidant properties of FPP seem not to be related to the presence of flavonoids or flavonoid-like compounds. Further studies are necessary to better characterize the active principal(s) of FPP in terms of its antioxidant properties.
Collectively, present data demonstrate that Fe-NTA plus H2O2 induce oxidative DNA and protein damage. Furthermore Fe-NTA/ H2O2 decreases antioxidant defenses, as indicated by a significant fall in GSH levels in Jurkat cells. FPP significantly blocked oxidative damage to DNA and proteins probably both due hydroxyl radical scavenging as well as iron chelating properties.
References
- Ogino T and Okada S: Oxidative damage of bovine serum albumin and other enzyme proteins by iron-chelate complexes. Biochim Biophys Acta 1245: 359-65, 1995.
- Anderson RL, Bishop WE and Campbell RL: A review of the environmental and mammalian toxicology of nitrilotriacetic acid Critical Reviews in Toxicology 15: 1-102, 1985.
- Li JL, Okada S, Hamazaki S, Ebina Y and Midorikawa 0: Subacute nephrotoxicity and induction of renal cell carcinoma in mice treated with ferric nitrilotriacetate. Cancer Res 47: 1867-9, 1987.
- Sarker AH, Watanabe S, Seki S, Akiyama T and Okada S: Oxygen radical-induced single- strand DNA breaks and repair of the damage in a cell-free system. Mut Res 337: 85-95, 1995.
- Iqbal M, Giri U and Athar M: Ferric nitrilotriacetate (Fe-NTA) is a potent hepatic tumor promoter and acts through the generation of oxidative stress. Biochem Biophys Res Cornm 212: 557-63, 1995.
- Iqbal M, Sharma SD, Zadeh HR, Hassan N, Abdulla M and Athar M: Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate (Fe-NTA) mediated hepatic injury. Redox Rep 2:385-91, 1996.
- Iqbal M and Athar M: Attenuation of iron-nitrilotriacetate (FeNTA)-mediated renal oxidative stress, toxicity and hyperproliferative response by the prophylactic treatment of rats with garlic oil, Food Chern Toxicol36: 485-95, 1998.
- Okada S, Hamazaki S, Ebina Y, Li 1L and Midorikawa O: Nephrotoxicity and its prevention by vitamin E in ferric nitrilotriacetate promoted lipid peroxidation. Biochim Biophys Acta 922: 28-33, 1987.
- Goddard JG, Basford D and Sweeney GD: Lipid peroxidation stimulated by iron nitrilotriacetate in rat liver, Biochem Pharmacal 35: 2381-7, 1986.
- Rice-Evans CA and Miller N: Antioxidant activities of flavonoids as bioactive components of food, Biochern Soc Transact 24: 790-95, 1996.
- Virgili F, Kobuchi H and Packer L: Procyanidins extracted from Pinus maritima (Pycnogenol): scavengers of free radical species and modulators of nitrogen monoxide metabolism in activated murine RAW 264.7 rnacrophages. Free Radic Bioi Med 24: 1120-9, 1998.
- Prajda N, Singhal RL, Yeh YA, Olah E, Look KY and Weber G: Linkage of reduction in 1-phosphatidylinositol 4-kinase activity and inositol 1,4,5-trisphosphate concentration in human ovarian carcinoma cells treated with quercetin. Life Sci 56: 1587-93, 1995.
- Csokay B, Prajda N, WeberG and Olah E: Molecular mechanisms in the antiproliferative action of quercetin. Life Sci 60: 2157-63, 1997.
- Osato JA, Korkina LG, Santiago LA and Afanas’ev IB: Effects of bionormalizer (a food supplementation) on free radical production by human blood neutrophils, erythrocytes, and rat peritoneal macrophages. Nutrition 11: 568-72, 1995.
- 15 Okuda D, Orniarni H, Zhou A, Osato A and Santiago LA: Studies on biological activities of Bionormalizer. Clin Rep 27: 4249-4258, 1993.
- Santiago LA, Uno K, Kishida T, Miyagawa F, Osato JA and Santiago LA: Effect of Bionormalizer on serum components and immunological functions in humans, Neurosciences 20: J 49-52, 1994.
- Kobuchi H and Packer L: Bio-normalizer modulates interferongamma- induced nitric oxide production in the mouse macrophage cell line RAW 264.7. Biochern Mol Bioi Int 43:141-52, 1997.
- Santiago LA, Osato JA, Liu J and MoriA: Age-related increases in superoxide disrnutase activity and thiobarbituric acid-reactive substances: effect of bio-catalyzer in aged rat brain. Neurochern Res 18: 711 -7, 1993.
- Santiago LA, Osato JA, Ogawa N and Mori A: Antioxidant protection of bio-normalizer in cerebral ischaemia- reperfusion injury in the gerbil. Neuroreport 4: 1031 -4, 1993.
- Marcocci L, D’Anna R, Yan U, Haramaki Nand Packer L: Efficacy of Bio-Catalyzer alpha.rho no. l1 (Bio-Normalizer) supplementation against peroxyl radical-induced oxidative damage in rat organ hornogenates. Biochern Mol Bioi Int 38: 535-41, 1996.
- Awai M, Narasaki M, Yamanoi Y and Seno S: Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am 1 Pathol 95: 663-73, 1979.
- Hunt JV, Simpson 1A and Dean RT: Hydroperoxide-rnediated fragmentation of proteins. Biochern 1 250: 87-93, 1988.
- Allison LA and Shoup RE: Dual electrode liquid chromatography detection for thiols and disulphides. Anal Chern 55: 8-12, 1983.