| Title | PRELIMINARY STUDY ON THE EFFECT OF BIO-NORMALIZER IN RODENT MALARIA |
|---|---|
| Year | |
| Author | Ma. Dorina Bustos, Fidelino Malbas, Jr.Joyce Sarah Ibana, Sherwin Galit, Rodolfo Perez, and Dr. James Akira Osato |
| Publisher |
BN Report 7/04
Preliminary Study on the Effect of Bio-Normalizer in Rodent Malaria
Ma. Dorina Bustos1, Fidelino Malbas, Jr.1 Joyce Sarah Ibana1, Sherwin Galit1, Rodolfo Perez1, and Dr. James Akira Osato2
1Research Institute for Tropical Medicine (RITM), Dept of Health, Manila, Philippines
2Osato Research Institute, Gifu University, Gifu City, Japan
Introduction
The evolution and rapid spread of drug resistant Plasmodium falciparum malaria in the last 40 years has diminished whatever available effective antimalarials exist in the treatment armamentarium worldwide. Multi-drug resistance is a real problem in South East Asia, and the worsening drug resistance in Africa accounts for high morbidity and mortality especially in the under 5 age group. Likewise, in low transmission areas, drug resistance also compromises vector control efforts instituted by malaria control programs. This necessitated R & D with other synthetic or naturally occurring products, as well as possible antimalarial drug combinations.
Bio-Normalizer, developed at the Osato Research Institute in Japan from fermented Carica papaya, is a hydroxyl radical (.OH) scavenger that acts as an immunomodulator, metal-ion chelator and free radical regulator (Santiago L, et al,, 1991, 1992). Numerous researches in the past 15 years attest to its efficacy as a potent immunomodulator which enhances the immune system (Osato JA et al,, 1995; Santiago L et al,, 1994), enhance the anti-oxidative capability of the liver (Koide Y et al,, 1993), inhibit lipid peroxidation (Okuda H et al,, 1993), and fight off infections (Osato JA et al,, 1994). It promotes optimum production of interferons and stimulates natural killer cell activity (Osato JA, 1995; Korkina LG, 1995; Kobuchi H and Packer L, 1997).
Pre-clinical studies in mice demonstrated significantly increased activity of natural killer (NK) lymphocytes against a variety of tumor, cancer and virus-infected cells as well as controlled hematogenous metastasis (Okuda H et al,, 1993). Other studies showed that it can help protect the heart against ischemia-reperfusion injury by lowering the leakage of enzyme lactate dehydrogenase (LDH) from ischemia-injured heart tissues in rats (Haramaki N et al,, 1995).
Other pre-clinical in vitro studies on bacterial isolates from rectal and gastric exudates of patients with enterocolitis, diarrhea and gastroenteritis showed antimicrobial activity of Bio-Normalizer, with 89% thinning of growth of isolates. These results suggest that BN has the potential to stop the growth of commonly encountered pathogens in the gastrointestinal tract (Osato, JA, Bernas G, Remo G et al,, 1994).
One clinical study in Papua New Guinea documented the product to be highly effective clinically against malaria (Wangi et al,, 2003, manuscript in preparation). The 5-day in vivo study looked at the safety and efficacy of 100 mg/kg/day Bio-Normalizer given over three days to patients with acute uncomplicated P. falciparum malaria. Results showed 15/16 (94%) Adequate Clinical and Parasitological Response (ACPR) by Day 4, with persistence of fever in one patient. It was a crude and rapid assessment of BN with a very small number of patients observed over 5 days, to form the basis for a bigger clinical trial.
In the absence of other pre-clinical or clinical studies against parasitic/protozoan infections using this product, this study was done to validate the efficacy of BioNormalizer in an animal model using BALB/c mice infected with Plasmodium berghei malaria.
Methodology
Mice were bred and reared at the Research Institute for Tropical Medicine (RITM) Veterinary Research Department. All safety precautionary procedures were observed for experimental infection, following the guidelines of and with approval from the Institutional Animal Care and Use Committee (IACUC) following the rules stipulated in Department of Agriculture Administrative Order No. 40.
Three groups of specific pathogen-free (spf) 6-week old BALB/c mice (5 per group) were prepared. Healthy mice of appropriate weight 16-18 gms, all female, were acclimatized for 1 week prior to inoculation. The animals were given treated clear water daily and the necessary veterinary care management, with each group caged separately and kept in a separate room to avoid any cross infestation of other animals in the veterinary lab.
This is the standard sample size of mice (6-8 per group) in preliminary animal experimentations. The 3 groups are:
Group 1: Control group – no treatment
Group 2: Treatment control group – received treatment with Chloroquine intra-peritoneal
Group 3: Treatment trial group – received treatment with Bio-Normalizer intra-peritoneal.
Preparation of parasite inoculum. Procedures for parasite preparation and mice inoculation or infection as described by Cox FEG, 1998, were followed. Cryo-preserved P. berghei was thawed using sterile 1.6% Na Cl (Baxter/Fenwal, 4B7870) at 37°C for 1-2 minutes. The infected blood was then transferred to 50-ml centrifuge tubes with a sterile Pasteur pipette. The blood volume=V was measured, and then added 0.1x V of 12% NaCl slowly, drop-wise, while shaking the tube gently. This was allowed to stand for 5 minutes. Repeat adding 10x V of 1.6% NaC1 slowly, dropwise, swirling the tube. After which the tube was centrifuged at 1,500 rpm at 20°C for 5 min.
The supernatant was aspirated and l0xV of malaria culture medium slowly added, drop-wise, while shaking the tube. The tube was again centrifuged at 1,500 rpm at 20°C for 5 minutes and the remaining supernatant aspirated. After resuspending pelleted blood cells in sterile PBS, a smear was immediately done to check parasite count and parasite viability.
Animal inoculation. Acclimatized 6-week old BALB/c were made ready, each mice weighed and tagged (as labels) on the day of inoculation. 100ul inoculum was injected intra-peritoneal immediately into a recipient mouse (106 infected erythrocytes per dose).The mice were returned to the cages and observed for 2-3 days. A tail-snip blood smear was done by Day 3 post-infection on each of the mice to ascertain if they have reached at least 0.5-1% parasitemia.
Counting parasitemia. A thin blood film from tail-snip blood was done, fixed with methanol for 2 minutes, and stained with Giemsa. Percentage infected RBC (100x oil immersion) was counted using this formula:
No. of parasites X 10,100,000
10-20 fields (250 RBC)
Treatment. When the mice manifested with clinical symptoms i.e. ragged fur coat, rigors, red eyes, and parasite count was more than 1% parasitemia, treatment was started.
Treatment dose:
Group 1: Control, no treatment
Group 2: 25mg/kg chloroquine intra-peritoneal dose/day x 3 days
(0.025 mg/g mouse)
Group 3: 100 mg/kg Bio-Normalizer intra-peritoneal dose/day x 3 days
(0.10 mg/g mouse)
Chloroquine 150mg base/tab is manufactured by Pharmamed Manila, distributed by IDA, The Netherlands. Bio-Normalizer 3 mg/sachet is manufactured by Bio-Normalizer Manufacturing Corporation, Sun-O International, Inc. Gifu, Japan.
Observation for parasitological treatment response. In all three groups of mice, blood smear by tail snip were done to monitor treatment response to Chloroquine and Bio-Normalizer on Days 0, 2, 4, and 7. Blood smear were also done on the Control Group on the same Days. Mice not responding to treatment (positive parasitemia) and manifesting with severe malaria after Day 8 were culled by intra-cardiac puncture under inhalation anesthesia and parasitized blood drawn out. All mice underwent intra-cardiac puncture under inhalation anesthesia post-experimentation.
Analysis
The parasitological endpoint parameter is number of days to clear parasites (by blood smear) during or after treatment.
Results
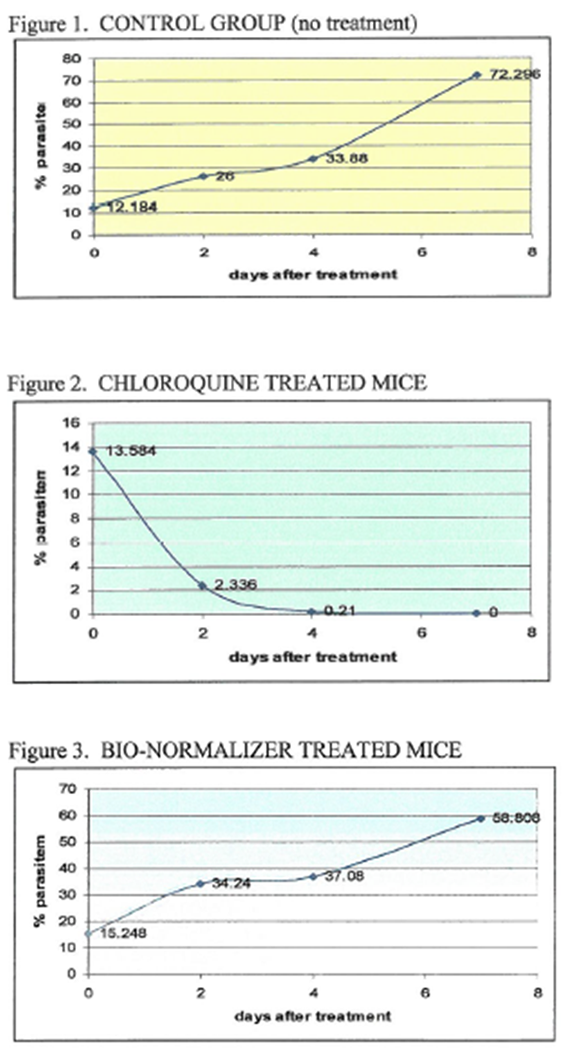
By the third day post-inoculation, mice manifested with clinical symptoms of malaria showing awkward appearance, ragged fur coat, rigors and red eyes. However, normal weight of all mice was maintained within the 7-day observation period (Table 1).
The infected mice manifested clinically by the 3rd day post-inoculation. Parasite count from tail-snip blood smears of the 15 mice (5 for each group) ranged from 1.2 to 17% parasitemia (Table 1). This was the Day 0 baseline parasitemia before treatment was started on Group 2 (CQ) and Group3 (BN) mice.
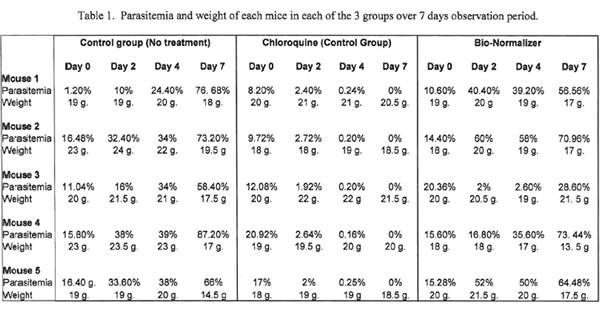
All mice in the control group showed a gradual increase in parasitemia until Day 7 (Figure 1). All mice treated with Chloroquine showed decreasing parasitemia by Day 2 and Day 4, and negative by Day 7 (Figure 2). In Group 3, except for mouse no. 3, other mice receiving Bio-Normalizer showed a gradual increase in parasitemia from Day 2 to Day 7 (Figure 3; Table 1).
Discussion
There is a clear trend of the anti-malarial effect of Chloroquine in the control CQ treated group. The anti-malarial effect of Bio-Normalizer was not observed in 4 mice. Mouse no.3 in Group 3 (BN) presented paradoxical results, with parasitemia going down on Days 2 and 4, with Day 7 count not as elevated as the other mice in the same group. This could be an immuno-modulating effect, initially suppressing parasite increase. This could also be attributed to manipulations, i.e. uneven homogenizing of BN granules in the stock solution or the syringe, though this was regularly shaken as needed.
Although no major conclusion could be drawn as to the anti-malarial effect of BN in rodent malaria with these very preliminary results, it is recommended that further dose finding studies be done i.e. increasing dose, boosting immune status with BN before infection, and longer treatment duration. Furthermore, BN could also be tried for treatment of dogs and other domestic animals manifesting with gastro-intestinal problems, i.e. vomiting, lack of appetite, convalescent stage.
Reference
Cox FEG. 1998. Major animal models in malaria research. In: Wernsdorfer WH, McGregor I, eds. Malaria: principles and practice of malariology. Vol 2. Ediburgh: Churchill Livingstone; 1998. P 1503-1543.
Haramald N, Marcocci L, D’Anna R, Yan LJ, Kobuchi Hand Packer L (1995). Bio Catalyzer □.□ No. 11 (Bio-Normalizer) Supplementation: Effect of oxidative stress on isolated rat hearts. Biochemistry and Molecular Biology International; 36 (6): 1263-1268.
Kobuchi H and Packer L (1997). Bio-Normalizer modulates Interferon gamma-induced nitric oxide production in the mouse macrophage cell line raw 264.7. Biochemistry and Molecular Biology International43 (1): 141-52, Sept 1997.
Koide Y, Miyagawa F, Santiago L, Osato JA and Mori A (1993). Effects of BioNormalizer on alcohol-induced changes in blood substances in humans. Neurosciences19 suppl 1: 85-88.
Korkina LG (1995). Effects of Bio-Normalizer on the clinical symptoms, free radical and immune status of patients with chronic viral hepatitis, diabetes mellitus and atopic diseases. 88th Annual Convention of the Philippine Medical Association, Manila, Philippines, 26 May 1995.
Okuda H, Ohminami H, Zhou A, Matsuura Y, Osato JA and Santiago LA (1993). Effect of Bio-Normalizer on lipid metabolism and liver injury repair. Clinical Report 27 (111):54-56.
Okuda H, Ohminami H, Zhou A, Matsuura Y, Osato JA and Santiago LA (1993). Effect of Bio-Normalizer on lipid metabolism and liver injury repair. Clinical Report 27 (111):54-56.
Okuda H, Ohminami H, Zhou A, Matsuura Y, Osato JA and Santiago LA (1993). Effect of Bio-Normalizer on natural killer cell activity in mice with tumor. Clinical Report 27 (11): 47-48.
Osato JA, Korkina LG, Santiago L and Afanas’ev IB (1995). Effects of Bio-Normalizer (a food supplement) on free radical production by human blood neutrophils, erythrocytes, and rat peritoneal macrophages. The International Journal of Applied and Basic Nutritional Sciences 11 (5); 568-572.
Osato JA, Bernas G, Remo G, Cuadra M, Urbano MS, Abrigo R, Santiago L and Takamizawa K (1994). Antimicrobial potential of Bio-Catalyzer □.□ No. 11 (Bio Normalizer) against enteric microorganisms. Acta Manilana 42: 9-14.
Peters W. Chemotherapy and Drug Resistance in Malaria, 2nd Edition. Academic Press Limited, London, 1987.
Santiago L, Osato JA, Hiramatsu M, Edamatsu R and Mori A. (1991). Free radical scavenging action of Bio-catalyzer α.p No. 11 (Bio-Normalizer) and its by-product. Journal of Free Radical Biology and Medicine 11 (4): 379-383.
Santiago L, Osato JA and Mori A. (1992). Stability of the Hydroxyl radical scavenging components of the health food “Bio-Normalizer”. Medical Science research 20: 27-28.
Wangi JK, Venudi JA and Auharai P (2003). Bio-Normalizer (BN) treatment outcome of uncomplicated malaria in National Capital District, Papua New Guinea. (manuscript in preparation) .