| Title | EFFECTS OF BIO-NORMALIZER ON THE EXPERIMENTAL WOUND HEALING |
|---|---|
| Year | |
| Author | Ludmila G. Korkina, Tatiana V. Snigireva, Galina A., Ibragimova, Irina B. Deeva, Tatiana K. Dubovaya, Anatoly, A. Dreval, and Igor B. Afanas’ev |
| Publisher |
R5
Effects of bio-normalizer on the experimental wound healing
Korkina LG, Snigireva TV, Ibragimova GA, Deeva IB, Dubovaya TK, Dreva AA and Afanas’ev IB
III. EFFECTS OF BIO-NORMALIZER ON THE
EXPERIMENTAL WOUND HEALING
Ludmila G. Korkina1, Tatiana V. Snigireva1, Galina A.
Ibragimova1, Irina B. Deeva1, Tatiana K. Dubovaya2, Anatoly
Dreval2, and Igor B. Afanas’ev1
1- Russian Institute for Pediatric Hematology, Leninskii pr 117, Moscow 117513, Russia
2- Russian Medical University, Dept. of Histology, Ostrovityanova 1A Moscow 117437, Russia
INTRODUCTION
Wound healing is a biological process of an utmost complexicity. It is characterized by a complex response of various cell types such as keratinocytes, fibroblasts, macrophages, basal cells, lymphocytes, polymorphonuclear leukocytes, etc. As a result, the wound defect transforms into a healed scar. At the first stage of wound healing, the inflammatory reaction on damaged tissue debris occurs. Inflammation is one of the most spectacular examples of free radical-mediated processes [ ]. A main source of oxygen free radicals in an inflammatory loci is believed to be phagocytozing cells attracting by necrotic products []. These very reactive species as well as some other mediators released from the inflammatory cells serve as the attractants for further inflammatory cell recruitment. At the same time, the mediators of inflammation, for example interleukin 1, leukotriene B4, platelet-activating factor, and some other cell growth factors are known to be the agonists of leukocyte oxidative burst by stimulating oxygen radical release from inflammatory leukocytes and tissue macrophages. Fibrosis occurs at the later stages when the connective tissue formation is taking place. Oxygen radicals released from cells can modulate the fibroblast growth and division and collagen fiber production. Therefore, the prooxidant/antioxidant balance in the healing wound area is very important for the rapid and non-impaired process of skin repair and scar formation. Since Bio-Normalizer (BN) is recognized as the natural health product possessing marked antioxidant properties [ ] and immunomodulating activity [ ], it can be applied topically or for the whole organism in order to diminish the inflammatory response and to accelerate the regenerative processes in the healed skin defect.
MATERIALS AND METHODS
Animal model of incision wound
Sixty male Wistar rats with weight ranging from 150 to 200 g were used in the experiments. The animals were kept under the standard laboratory conditions and supplied with chow food and water ad libitum. All the experimental procedures have been performed in accord with Intemational Guidelines concerning the studies in which the experimental animals are used.
A back side part of the rat head was shaved and the epilated skin was sterilized with 70% alcohol and 5% iodine solution and was snipped off with surgical scissors through all the layers: keratinous, spinous, basal cell, cutis, and adipose ones. An incision wound size was approx. 1.5x 1.5 cm (Photo 1). All the procedures with animals were carried out under nembutal narcosis.
The animals with incision wound were divided into 4 experimental groups each consisting of 12 rats. The rats of Group 1 were kept without any treatment (control). In Group 2 rats BN was applied topically at the wound surface immediately after incision and 3 more times within 3 following days. The animals of Group 3 were given BN with food during 7 days before operation. Group 4: BN was given with food during 7 days after operation. The animals were sacrificed by intraperitoneal injection of 60 mg/kg of nembutal on 3d, 10th, and 15th post-operative days.
Histopathological analysis.
The wound surface size was estimated according to the follow procedures: a sterile sheet of the transparent polyvinyl film was put on the damaged surface and the wound borders were drawn. The area inside drawn line was measured planimetrically. A size of wound area in the control group was assumed as 100% for each experimental point. The results were expressed as percentage of control values. To prepare material for histopathological examination, a 10×10 mm piece of skin was removed from the incision area, fixed in 10% potassium phosphate buffered neutral formalin, and dehydrated into series of alcohol solutions of the increasing concentrations. The dehydrated specimens were embedded in paraffin for ultrathin section preparation. The 6 µn vertical sections were prepared and stained by hematoxylin and eosin. Some of the sections were stained specifically to evaluate the collagen fiber deposition. The stained histological specimens were examined by using light microscopy.
Free radical status measurements
Oxygen radical production by circulating blood leukocytes was measured by luminol- and lucigenin-dependent chemiluminescence. Blood was collected from the rat tail vein. A fresh blood sample was layered onto the top of Dextran-metrizoate gradient for erythrocyte sedimentation. After 30 min incubation, the leukocyte-rich plasma was transferred in the centrifuge tubes and centrifuged at 300xg for 10 min. Cell pellets were resuspended in 10 ml of a cold Hank’s balanced salt solution (HBSS) and washed twice. Finally, washed leukocytes were resuspended in HBSS supplied with 5% fetal calf serum and stored at 4°C during analyses. Cell content was determined by hemocytometer and differential cell count was carried out microscopically in the stained cell smears. Usually the leukocyte suspension consists of 70% neutrophils, 20% lymphocytes, 8% monocytes. The contamination was negligble and did not affect further measurements. Cell viability was confirmed by the exclusion of trypan blue and was higher than 95%.
Chemiluminescence measurements were performed on a LKB luminometer mod. 1251 equipped with IBM-compartible computer for the automatic maintenance of the experimental conditions and for storage and mathematical analysis of data. In the course of experiment, 50 µl of cell suspension and 950 µl of HBSS with 50 µl luminol or lucigenin preheated at 374°C were placed in the polysterene vial. This vial was transferred in the thermostate chamber of a luminometer and incubated there at 37°C and continuous mixing for 5 min. The spontaneous CL intensity was recorded continuously, and the maximal value was determined. Then, 50 µl of 0.1% latex particle suspension were dispensed automatically into cell-containing mixture and the enhancement of CL intensity (CL response) was monitored for 15 min. Again, maximal value of the activated CL was fixed at the luminometer-build computer. The amplitude of the CL-response was estimated as a difference between the maximal intensities of activated and spontaneous CL. Each measurement was performed in triplicates, and variabilities between values did not exceed 10%. The results were presented as mean ± SEM in mV/106 cells.
The reduced glutathione content in the erythrocytes was determined according to the Beutler method [ ]. The erythrocytes were obtained after dextran-metrizoate sedimentation. They were washed three times in 0.1 M potassium phosphate buffer, pH 7.4 and and finally adjusted to the 2% of hematocrit.
RESULTS AND DISCUSSION
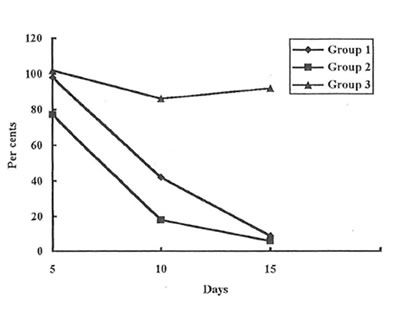
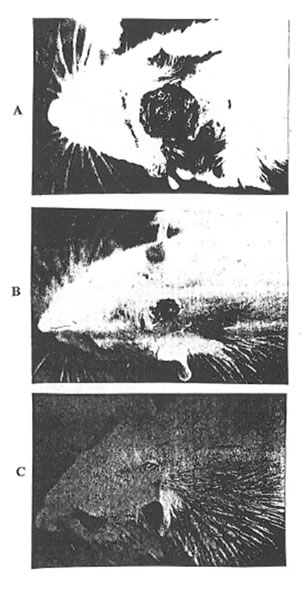
As it was determined visually (Photo 1 A, B, and C) and estimated quantitatively (Fig. 1) the wound covering in the rats of Group 2 was much faster at the first stages of healing process than in the rats of Group 1 and 3. (Similar results were obtained for the animals of Group 4. Data are not shown.). Even on the 3rd day after incision, the rats of Group 2 exhibited some features of skin regeneration such as comparatively weak inflammation with a small amount of the transparent serose exsudate, tissue granulation, and the beginning of the wound epitalization. On the 10th day the area of the open wound was much less in Group 2 animals comparing with control and Group 3 and 4 ones. By the end of the observation period the wounds were practically covered and their size became equal for all the groups studied.
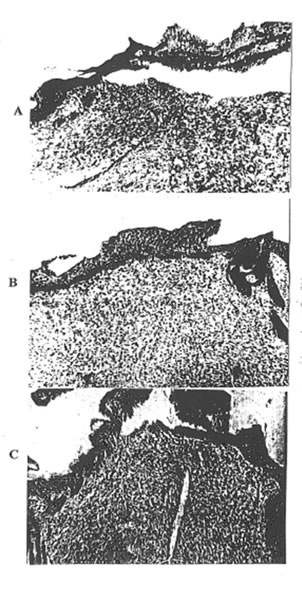
On the 3rd day after wounding, the histopathologic changes in rats of Group 1 were consistent with those for severe traumatic inflammation and were as follows: the surface of the wound was covered with necrotic debris. There was an acute severe serohemorragic inflammation with the multiple areas of irregular size and shape of coagulative necrosis.
The epidermis showed spongiosis and invasion by leukocytes. There was a marked neutrophilic infiltration, extending down to the deeper structures and involving the subcutaneous tissue. There was also a considerable intercellular and intracellular edma in the cutis (Photo 2A and 3A). By that time, all the features of the acute cutaneous inflammation mentioned above were presented but to the much lesser extent in the rats of Group 2 (Photo 2B and 3B). There was mild infiltration with macrophages, round nuclear cells and fibroblasts. As is seen from Photo 3B, the first signs of skin repair such as fibroblast activation and thin collagen fiber formation were presented. Contrary, there was a very intensive inflammatory reaction with huge leukocyte and macrophage infiltration in the rats of Group 3. The infiltration was predominantly of polymorphonuclear leukocytes, which penetrated upper and deeper skin layers. The distraction of elastic tissue was seen where the infiltration occurred. There was significant dilation of blood and lymphatic vessels with the signs of extravasation of leukocytes and erythrocytes. In addition, a strong exsudative reaction was observed (Photo 2C). In the deeper layer of the connective tissue, there was a moderate inflammatory reaction.
there was Histopathological picture of the development of reparative process in the rats of Group 1 on 10th day after the experiment beginning is presented in Photo 4A. There were tissue granulation, epitelium restoration, and fibrosis activation. However, the local inflammatory spots still remained, the process of epitelization proceeded rather slowly, and therefore, the thickness of new-formed epitelium was insuflicient and irregular. Similar results were obtained for animals of Group 4. (Data are not shown.) At the same time, the very intensive regeneration of incision defect in the Group 2 rats was found. There was the formation of the mature collagenous fibers with a compact regular deposition. This new connective tissue was covered with a thick regular layer of epitelium in which the remodeling of hear folliculi was observed (Photo 4B). Histopathological picture of the Group 3 rats showed the presence of the inflammatory loci with neutrophilic infiltration and necrotic debtis. The very slow epitelization was obserwd (Photo 4C).
By the end of observation (15th day), the histopathology of incision defect in the rats of Group 1 and 3 was quite similar (Photo 5A and 5C, 6A and 6B). On a whole, the processes of connective tissue and scar formation were practically completed. However, the inflammatory and necrosis loci still presented. Furthermore, the epitelium did not cover all the wound defect, its thickness remained insufficient, and the essential skin structures like hair folliculi did not form. In the Group 2 animals, a very gentle and practically invisible scar was formed by 15th day. For these rats, the very fine picture of the connective tissue formed was observed: it contained the body of mature collagenous fibers in a close weave parallel to the surface of the epidermis. Between the mature collagenous fibers were the networks of elastic fibers. A large amount of the long and bipolar fibroblasts followed the lines of the collagenous fibers. This new connective tissue was very well vasculated, and the blood vessels were oriented perpendicular to the skin surface. A mature epitelium covered all Ute wound defect. It seems to be very important that in this case there was a very tough conjunction between dermal and epidermal layers.
Studying the free radical status in the blood of the experimental animals, we found that the process of wound healing was accompanied with the characteristic changes of the luminal- dependent CL (Table 1). The rats of Group 1 exhibited very intensive spontaneous and latex-activated CL with a maximal value on the 10th day after incision wounding. Topically given BN (Group 2) was a very effective inhibitor of both spontaneous and activated luninol-amplified CL, especially at the first stages of the reparative process (3rd and l0th days). BN given per as before and after incision (Group 3) was a little less potent as an inhibitor of leukocyte CL on 3rd and 10th days. In contrast, there was a suppression of lucigenin-amplified CL on 3rd and l0th days in the control Group (Table 2). In the rats of Group 2 there was practically full restoration of the normal lucigenin amplified CL by the 10th day. For the animals of Groups 3 and 4 such a restoration was found on 15th day only that was similar to those of control Group. The content of reduced GSH in the erythrocytes of control rats (Group 1) was significantly increased comparing with normal value on the 10th and 15th days & after incision (Table 3). Meanwhile, BN applied both topically or to the whole organism decreased glutathione level down to the normal values.
On the basis of the results obtained, we have made the following CONCLUSIONS:
1. Topically applied BN significantly accelerated the wound healing process. It improved simultaneously three stages of skin regeneration: BN suppressed the traumatic inflammation, stimulated the connective tissue formation, and accelerated the epitelia layer formation.
2. BN applied to the whole organism did not affect the reparative processes.
3. BN applied topically suppressed oxidative stress induced by the skin incision, while BN applied to the whole organism was a less effective inhibitor of oxidative stress.
4. BN may be regarded as a very promising medicine for topical application in the treatment of incision wounds.
Table 1. Intensities of the luminol-dependent chemiluminescence (CL) in the blood leukocytes.
|
Experimental |
Time after incision, days |
Intensity of spontaneous CL, mV |
Intensity of latex- activated CL, mV |
|
Intact animals |
– |
58 |
356 |
|
Group 1 |
3 10 15 |
331 440 111 |
4406 16640 3016 |
|
Group 2
|
3 10 15 |
289 112 54 |
1384 2148 1432 |
|
Group 3
|
3 10 15 |
557 130 57 |
3673 6643 773 |
|
Group 4 |
15 |
69 |
1505
|
Table 2. Intensities of lucigenen-dependent chemiluminescene (CL) in the blood leukocytes.
|
Experimental |
Time after incision, days |
Intensity of spontaneous CL, mV |
Intensity of latex- activated CL, mV |
|
Intact animals |
– |
16 |
196 |
|
Group 1 |
3 10 15 |
6 7 15 |
12 58 62 |
|
Group 2
|
3 10 15 |
11 8 30 |
26 148 153 |
|
Group 3
|
3 10 15 |
8 7 18 |
22 75 183 |
|
Group 4 |
15 |
33 |
190 |
Table 3. Reduce glutathione concertration in the erythrocytes (µmol/g Hb).
|
Experimental |
Time |
after |
Incision |
Days |
|
Group 1 |
9.60±0.8 |
8.54±0.9 |
12.84±1.1 |
10.69±1.2 |
|
Group 2 |
9.60±0.8 |
9.25±1.2 |
10.37±0.8 |
8.54±0.9 |
|
Group 3 |
8.85±0.6 |
8.50±1.1 |
10.90±1.2 |
10.11±0.8 |
|
Group 4 |
9.60±0.8 |
n.a |
n.a |
8.43±0.7 |
Legends to photos and Figures.
Photo 1A. An overall view of the incision wound (0 day, for all Groups).
Photo 1B. An overall view ofthe healing wound (10th day, Group 1).
Photo 1C. An overall view of the healing wound (10th day, Group 2).
Photo 2. The histopathological picture of the skin defect on the 3rd day after operation: A – Group 1; B- Group 2; C- Group 3 ( x 63).
Photo 3. The histopathological picture of the skin defect on the 3rd day after operation: A – Group 1; B – Group 2; C – Group 3 ( x 400).
Photo 4. The histopathological picture of the skin defect on the 10th day after operation: A -Group 1; B- Group 2; C – Group 3 ( x 63).
Photo 5. The histopathological picture ofthe skin defect on the 15th day after operation: A – Group 1; B- Group 2; C- Group 3 ( x 63).
Photo 6. The histopathological picture of the skin defect on the 15th day after operation: A- Group 2; B – Group 3 ( x 400).
Fig. 1. Dynamics of the wound heating (percentage of the values for the control group).