| Title | Inhibition of hippocampal K+-stimulated L-[3H] aspartate release by Bio-normalizer |
|---|---|
| Year | |
| Author | Librado A. Santiago, James Akira Osatol, Ikuo Kinno and Akitane Mori |
| Publisher |
Inhibition of hippocampal K+-stimulated L-[3H] aspartate release by Bio-normalizer
Librado A. Santiago, James Akira Osatol, Ikuo Kinno and Akitane Mori
Department of Neuroscience, Institute of Molecular and Cellular Medicine, Okayama University Medical School, 2-5-1 Shikata-cho, Okayama 700, Japan; lSun-O International Inc., Gifu, Japan
Running head: hippocampal K+-stimulated aspartate release
Key words: potassium-stimulated aspartate release; monoamine release; hippocampal slices; epileptic seizure; ischemia-reperfusion injury; Bio-normalizer
Corresponding Author:
Akitane Mori, M.D., Ph.D. Chairman, Dept. of Neuroscience
Institute of Molecular & Cellular Medicine
Okayama University Medical School
2-5-1 Shikata-cho, Okayama 700, Japan
Tel. 086-223-7151 (ext. 2643)
Fax 086-234-2426
Abstract
The potassium-stimulated L-(3H] aspartate release from hippocampal slices of ddY mouse was significantly inhibited by pretreating the tissue with 0.05% (w/v) of Bio normalizer, a natural fermented health food product with hydroxyl radical scavenging action. The modulation of aspartate release in the hippocampus indicates a possible involvement of antioxidant against synaptic excitation and excessive neuronal depolarization attributable to aspartate and mediated by an increased extracellular potassium ion.
Introduction
L-Aspartate (L-Asp), a naturally occurring amino acid transmitter present in substantial concentrations in the central nervous system, causes powerful excitation of neurons.1 It was shown that as a neurotoxin, L-Asp evoked a cortical spreading depression accompanied by changes in extracellular conductivity2 and produced membrane depolarization3 resulting to convulsive disorder,4 epileptiform discharge,5 and ischemia reperfusion injury.6 The excitatory release of L-Asp from rat hippocampal slices was attributed to free radical formation7 which participates in the genesis of ischemia-induced neuronal damage6,8 and development of epileptiform activity.9 Reactive oxygen species such as superoxide, peroxyl and hydroxyl radicals and hydrogen peroxide have been shown to be generated during brain injury.IO,ll Furthermore, the elevations of extracellular potassium which reflect the level of excitation, and the decrease in extracellular calcium, which reflects calcium entry into the cells can produce vital changes on the aspartate release at the presynaptic site of the neurone.l2 Pretreating the animals with vitamin E and selenium,13 glutathione reductase, superoxide dismutase and catalase,l4 have been demonstrated to prevent brain damage during epileptic seizures and ischemic injury.
Bio-normalizer is a product of the fermentation of papaya, other tropical herbal plants, and cereals from traditional Japanese foods, and has been reported to give many health benefits in preventing chronic diseases and in generating a feeling of well-being. In particular, Bio-normalizer has been found effective as a prophylactic agent in convulsive disorder, epilepsy in children and in various forms of myocardial, gastric and cerebral ischemic attacks. A proven scavenger of hydroxyl radical, Bio-normalizer was demonstrated to inhibit the thiobarbitric acid-reactive substances (TEARS) in the cortex, 15 and the dopamine and serotonin in the intrastriatal perfusate, 16 of rats intracortically injected with Fe3+ solution. Bio-normalizer also produced a significant reduction in ischemia-reperfusion injury-mediated increase in carbon-centered radicals and TEARS in the cortex, the hippocampus and the striatum of the gerbils.17
Our working hypothesis was that free radical formation, hyperactive release of monoamines and the generation of excitatory amino acid neurotransmitters may cooperate in the genesis of post-traumatic epilepsy, seizures and brain ischemia and may lead to neuronal death. These three interrelated events may be inhibited through antioxidant treatment such as Bio-normalizer. We developed an in vitro system to study the release of aspartate from the hippocampus, a brain region particularly sensitive to ischemic insults18 and seizures.l9
Materials and Methods
Materials
Radiochemically pure L-[3H] aspartate (specific activity: 843.6 GBq/mmol) was purchased from Du Pont Co. Biotechnol. Systems, Wilmington, DE, USA, and stored at 4oC. Bio-catalyzer α.p No.II (Bio-normalizer) was supplied by Sun-0 International Inc., Gifu, Japan. All other reagents were from Wako Pure Chern. Ind. Ltd., Osaka; Nacalai Tesque Inc., Kyoto; and Katayama Chern… Osaka, Japan.
Preparation of mouse hippocampal slices.
Male ddY mice (Japan SLC Co., Ltd., Hamamatsu, Japan) aged 10-15 weeks were 0 housed at 25 C and humidity of 50 ± 5% and were allowed free access to water and standard diet (Oriental Yeast Co., Ltd., Tokyo, Japan) . After cervical dislocation, their hippocampi were rapidly removed and plunged into ice-cold Krebs-Ringer-bicarbonate buffer (in mM: NaCI, 124; KCI, 3.5, KH2P04, 1.2; MgS04 7H20, 1.3; CaCI2, 1.2; NaHC03, 25; and glucose, 10), pH 7.4. About 18 mg each of hippocampus was cut twice transversely into slices (30 m) with a McIIwain tissue chopper and dipped into Krebs-Ringer bicarbonate buffer previously gassed with 95% 02/5% C02 for at least one hr…
In vitro experimental model: Release experiments.
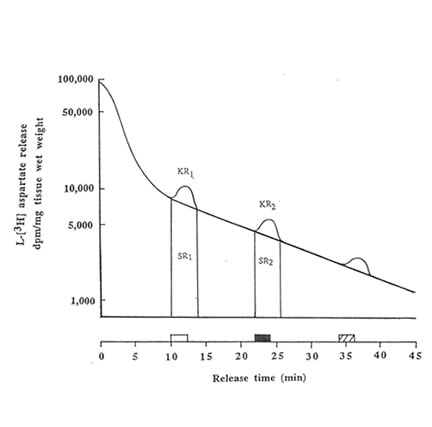
The hippocampal slices were preincubated for 5 min at 370C in previously gassed standard buffer solution in order to allow functional recovery, and thereafter loaded with L [3H] aspartate (final cone: 500 μ M) for 30 min at 37oC. In stimulated buffer, NaCl was replaced by equimolar amount of 50 mM KCl (to make a total concentration of NaCl + KCl equivalent to 127.5 mM) as the hyperosmotic solution. The hippocampal slices were transferred to a stainless cell chamber (stainless syringe holder KS-13, Advantec, Tokyo, Japan) by filtration, immersed in a water bath at 37OC and perfused initially with the normal buffer medium. The release of radioactivity accumulated by the cells was monitored every 30 s at a flow rate of 1.5 ml/min for 45 min in 90 tubes. The selection of the perfusing medium namely: standard buffer (1); stimulated buffer containing 50 mM K+(2); stimulated buffer containing Bio-normalizer (3) was done by operating the five-way cock. The K+– induced aspartate release was done in three sequential phases(a) spontaneous release into normal medium (at 10-12 min, basal phase); (b) release induced by modified medium (at 22-24 min, stimulation phase); and (c) spontaneous release into standard medium (at 34-36 min, recovery phase). Two ml of counting scintillant was added to each fraction before subjecting the radioactive mixture to Liquid Scintillation Counter 703 (Aloka, Tokyo, Japan). The spontaneous release (SRI) of aspartate was estimated as the area under the baseline of peak I (KR1), while the potassium-stimulated asparate release (SR2) was estimated as the area under the baseline of peak 2 (KR2). The ratio of the spontaneous aspartate release was calculated as rSR = SR2/SR1 whereas the ratio of the K+-stimulated release was estimated as rKR = KR2/KR1. Peak 3 indicates the recovery phase which was used to evaluate the viability of the hippocampal slices after the stimulation.To test for the antioxidant activity of Bio-normalizer on the excitatory release of aspartate, different concentrations (0.05, 0.1 and 1.0% w/v) of Bio-normalizer were mixed separately in the stimulated buffer medium. The statistical significance of difference between the experimental groups was evaluated by U-test.
Results
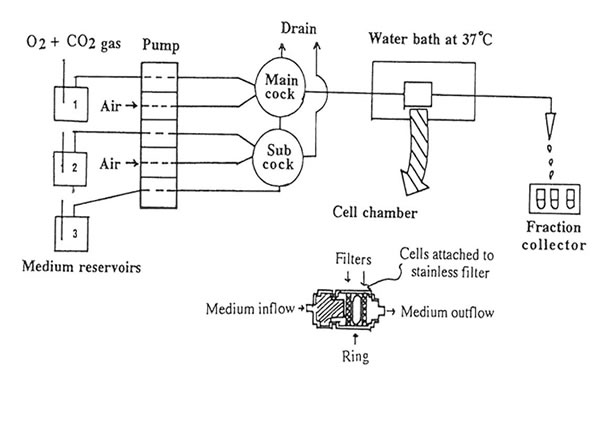
To elucidate the mechanism of excitatory amino acid neurotransmitters in brain injury, we developed an in vitro model of simulated K+-stimulated aspartate release from the mouse hippocampal slices. Figure 1 shows the schematic diagram of the release experiment. Using a five-way cock, the selection of the gassed buffer medium (normal or stimulated buffer with and without Bio-normalizer) could be allowed to perfuse one after the other into the slices. Air inlet was important in the set-up to allow for at least one cm distance between the buffers during buffer change. Extra care was taken in order not to incorporate much air when turning or moving the cock because bubbles tended to disrupt the medium flow rate causing fluctuations on the radioisotope readings in the perfusate. The slices were allowed to pass through a stainless filter, pressed by another stainless filter, separated by a polyethylene ring and rested in the cell chamber. The slices trapped in the cell chamber were continuously perfused with gassed buffer medium under a water bath (37o C) at a flow rate of 1.5 ml/min. Fractions were collected every 30 sin 90 tubes for 45 min.
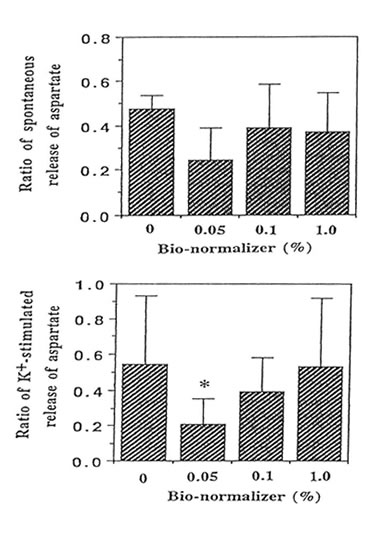
We performed the aspartate release experiments on individual mouse hippocampus (n = 11 or 12 animals per group). Figure 2 shows that the spontaneous efflux of L-Asp from hippocampal slices preloaded with L-[3H] aspartate was fast, about 50, 25, 10, 5 and I% of the accumulated radioactivity being released in 1, 3, 14, 25 and 45 min, respectively. Upon depolarization of the slices by increased concentration of extracellular K+, three peaks were observed at 10-12 min, 22-24 min and 34-36 min; the last peak showed broadening. Pre-experiments revealed the absence of radioactive substances in Bio-normalizer that may interefere with the tritium release experiments (data not shown). Addition of 0.05, 0.1 and 1.0% of Bio-normalizer in the K+ stimulated buffer modulated the hippocampal aspartate release. A significant decrease was observed using 0.05% Bio-normalizer (p < 0.001). With the presumption that the particulates when they adhered to the slices will tend to shrink or collapse and caused lowered aspartate release, we filtrered the insoluble particles in 1.0% Bio-normalizer.(Figure 3).No significant difference on the aspartate release was observed between filtered and unfiltered 1.0% Bio-normalizer solution (data not shown). As the inbitory action of Bio-normalizer on aspartate release decreased with increasing concentration of Bio-normalizer, the hyperosmotic pressure in Bio-normalizer solution at relatively higher concentrations may have interfered with its inhibitory action on the release of aspartate.
Discussion
The present paper represents the first report of a decrease in potassium-evoked [3H] aspartate release from hippocampal slices using a non-drug preparation Bio-normalizer. This experiment was designed because Bio-normalizer (a health food) was shown previously to: (1) scavenge hydroxyl radicals,15 (2) inhibit membrane lipid peroxidation in epileptic rats,l5 (3) modulate the excitatory release of monoamines (dopamine and serotonin) and their metabolites in the same model of epileptic rats,16 and (4) suppressed the carbon centred radicals and TBARS in post ischernic gerbils.l7 These observations reflect a unifying concept that Bio-normalizer acts as an antioxidant and thus is capable of inhibiting the toxic reactive oxygen species (such as superoxide, peroxyl and hydroxyl radicals and hydrogen peroxide) and the free radical reactions such as lipid peroxidation in the event of brain damage. Antioxidant therapy by vitamin E, selenium, glutathione reductase, superoxide dismutase and catalase,l3,14,20 among others have been also found useful in such brain disorders.
The formation of free radicals during ischemia-induced neuronal damage6,8 and seizures9 is believed to trigger the release of substantially greater amount of excitatory amino acid neurotransmitter aspartate. Kitagawa K. eta al. have shown that free radical formation follows brief ischemic episode and leads to onset of delayed neuronal death.21 By intracerebral microdialysis, Benaviste H. et al. have indicated elevated hippocampal extracellular aspartate during transient cerebral ischernia.22 The synaptic release of aspartate, which could have facilitated the seizures19,20 and ischemia- reperfusion injury ,8,23 is greatly influenced by the increment in the concentration of extracellular K+.24 Concomitantly, the increased levels of brain extracellular K+ may cause firing off monoamines like dopamine and serotonin, 25changes in membrane potentials and active transport of ions such as Ca++ shifts12 which effect significant changes in neuronal excitability and synaptic transmission. For instance, influx of intracellular “free” Ca++ might lead to availability of transition metal ions, and hence increased rates of lipid peroxidation.23 While the mechanism of action of Bio-normalizer on Ca++ transport in the neurones has yet to be established, our present data fully support of the antioxidant protection of Bio-normalizer in inhibiting the K+-evoked presynaptic release of putative excitatory amino acid aspartate in the hippocampus, a brain region particularly sensitive to ischemic insultsl8 and seizures.l9 We believe that Bio-normalizer interferes with the peroxidation of neuronal membrane and long term changes in excitatory neurotransmission (monoamines, excitatory amino acids and metal ions release); and this may be worth prophylactic intervention in epileptic seizures and ischemia-reperfusion injury.
Conclusion
In the present study, we have successfully used an in vitro model to simulate the excitatory release of L-aspartate from the hippocampus as mediated by an increased concentration of extracellular potassium, that is known to occur in vivo following intracortical injection of iron solutions and in brain damage during ischemia-reperfusion injury. The excessive stimulation of neurotransmitters and production of toxic reactive oxygen species in the brain may be significantly inhibited by pretreating the brain tissue with Bio-normalizer, a natural fermented health food product with potent hydroxyl radical scavenging activity.
References
- McLennan H. Blockade of excitatory amino acid transmitteres and epil epsy. In: Excitatory Amino Acid Transmission. Hicks TP LodgeD, McLennan H, eds. New York: Al an R. Liss, I nc. 1987: 1-18.
- Purpura DP, Girado M, Smith TG et al. J. Neurochem. 3, 238-268 (1959).
- Van Harreveld A and Ochs S. Am. J. Physiol. 189, 159-166 (1957).
- Weitz MD, Merlob P, Amir J et al. Arch. Neurol. 38, 258-259 (1981).
- Van Gelder NM and Courtois A. Brain Res. 43, 477-484 (1972).
- Pellegri ni-Giampietro DE, Cherici G, Alesiani M et al. J. Neurosci. 10, 1035-1041 (1990).
- Pellegrini-Giampietro DE, Cherici G, Alesiani M et al. J. Neurochem. 51, 1960- 1963 (1988).
- Oliver CN, Starke-Reed PE, Stadtman ER et al. Proc.Natl. Acad. Sci. USA 87,5144-5147 (1990).
- MoriA, Yokoi I and Kabuto H. Effects of vitamin E and its derivatives on posttraumatic epilepsy and seizures. In: Vitamin E in health and diseases. Packer L, Fuchs J, eds. New York: Marcell Dekker, Inc., 1992: 851-858.
- Hall ED, Andrus PK and Yonkers PA. J. Neurochem. 60, 588-594 (1993).
- Mori A, Hiramatsu M, Yokoi I et al. Pavlov. J. Biol. Sci. 25, 54-62 (1990).
- Rice ME and Nicholson C. Neuroscience 38, 295-310 (1990).
- Willmore U and R ubin JJ. Neurology 3, 63-69 (1981).
- Singh Rand Pathak DN. Epilepsia 31, 15-26 (1990).
- Santiago LA, Osato JA, Hiramatsu M et al. Free Radical Biol. Med. 11, 379- 383 (1991).
- Santiago LA, Osato JA, Kabuto H et al. Med.Sci.Res. 21, 139-141 (1993).
- Santiago LA, Osato JA, Ogawa N et al. Neuro Report 4 (in press).
- Smith ML, Auer RN and Siesjo BK. Acta Neuropathol. 64,319-332 (1984).
- Janjua NA, Mori A and Hiramatsu M. Epilepsy Res. 6, 215-220 (1990).
- Ryan GP, Hackman JC and Davidoff RA. Neurosci. Lett. 44, 161-166 (1984).
- Kitagawa K, Matsumoto M, Oda T et al. Neuroscience 35, 551-558.
- Benveniste H, Drejer J, Schousboe A et al.J. Neurochem. 43, 1369-1374.
- Halliwell B and Gutteridge GMG. Free Radical Biology and Medicine. Oxford: Clarendon Press, 1989.
- Hackman JC, Ryan GP and Davidoff RA. Neurosci. Lett. 33, 289-293 (1982).
- Matos FF, Rollema H and Basbaum AI. Brain Res. 528, 39-47 (1990).
Figure Legends:
FIG.l. Schematic diagram of the in vitro continuous measurement of L-[3H] aspartate release from the hippocampal slices. The delivery of the gassed medium buffer at a flow rate of 1.5 ml/min to the cell chamber (voulme: 0.15 cc) and the detection of released aspartate are described under Materials and Methods.
FIG.2. Effects of 50 mM KCI on the L-[3H] aspartate release from hippocampal slices (30μm) preloaded for 30 min with 500 μM L-[3H] aspartate. Single representative experimental run is shown. The release of radioactivity accumulated by the cells constitutes three phases namely: basal phase ( ![]() ), stimulation phase (
), stimulation phase (![]() ) (
) (![]() )
)
FIG.3. Effect of different concentrations of Bio-normalizer on the high K+-induced release of L-[3H] aspartate from hippocampal slices (30 μM) preloaded with 500 μM L-[3H] aspartate. The number of experimental animal per group was 11 or 12. Significance of difference from control: * p < 0.001.The ratio of spontaneous release of aspartate (rSR) = SR2/SR1 while the ratio of the K+-stimulated release (rKR) = KR2/KR1.
Figure 1
Santiago et al
Figure 2
Santiago et al.
Figure 3
Santiago et al